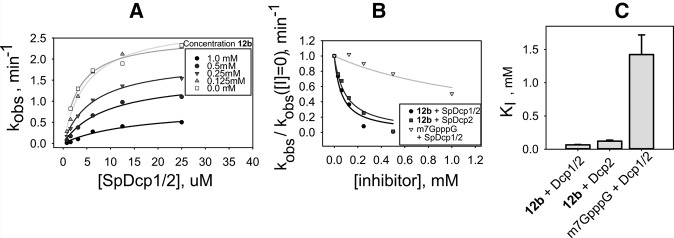
FIGURE 4.
Single-turnover inhibition kinetics of 12b with SpDcp1/2 and a 29-nt native-capped RNA substrate. (A) Fits to determine KMapp and kmax at different inhibitor concentrations; KMapp increases by fivefold and kmax decreases by threefold in the presence of 1 mM inhibitor, demonstrating that 12b is a mixed inhibitor of Dcp1/2 (see Supplemental Table S1). (B) Fits to Equation 1 of normalized rate data versus inhibitor concentration to determine KI under subsaturating enzyme concentrations. (C) Bar graph of determined KI values for 12b with Dcp1/2 or Dcp2 and m7GpppG with Dcp1/2; nucleotide 12b binds with ∼20-fold tighter affinity than m7GpppG.