Fig. 1.
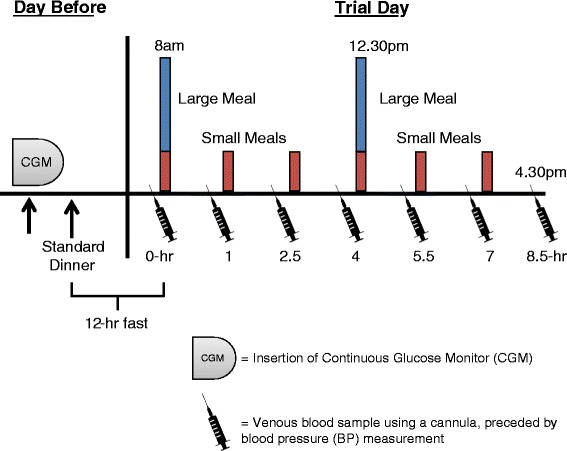
Study Protocol: For each dietary condition, participants came to the clinical trial facilities on 2 consecutive days. The day before the main trial day was for the insertion of a continuous glucose monitor and the consumption of a standardized evening meal. On the main trial day, BP and venous blood samples were collected at regular intervals, as shown, for up to 8.5 h following the first meal of each dietary condition
