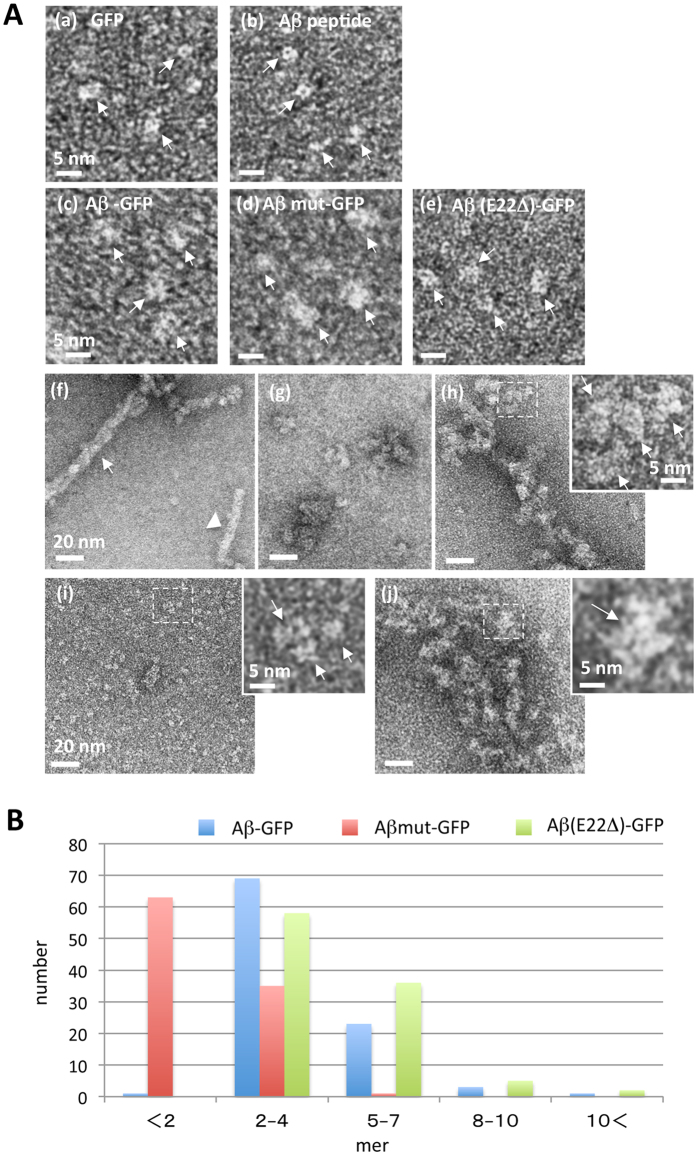
Figure 4. EM analysis of molecular feature of Aβ-GFP fusion proteins.
EM images (A) and analyses (B) of Aβ-GFP fusion proteins. GFP (a), monomeric Aβ peptide (b), Aβ-GFP (c), Aβmut-GFP (d), and Aβ (E22Δ)-GFP (e) are indicated by arrows in each panel. 24 h after incubation at 4 °C (pH8.5), Aβ peptide formed long fibrils (f) but Aβ-GFP (g,h) and Aβ (E22Δ)-GFP (j) formed oligomers with various sizes (g,j) or filamentous-looking aggregates (h). Almost all the Aβmut-GFP remained as small particles in the size of a monomer or a very small oligomer (i) without a clear sign of polymerization. The inset shows a magnified view of the dotted rectangle in (h–j) revealing single units of Aβ-GFP fusion protein oligomers (arrows). Measurement of the area of each unit (B) shows that a single unit of polymerized Aβ-GFP and Aβ (E22Δ)-GFP contains two to four molecules but the particles observed with Aβmut-GFP contain single to two molecules (n = 100 units). Scale bars: 5 nm (a–e) and insets), 20 nm (f–j).