Fig. 5.
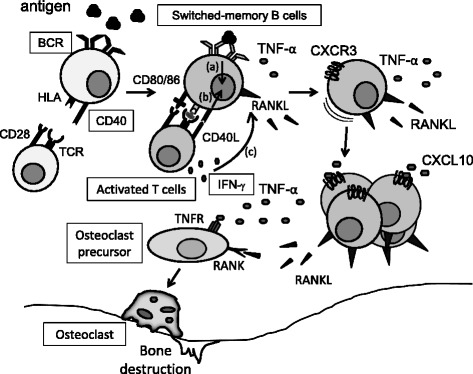
The current hypothetical model in this study. T cell-dependent responses play a pivotal role in B cell-derived receptor activator of nuclear factor kappa-B ligand (RANKL) expression in RA. Activation via (a) B-cell receptor (BCR) and (b) CD40 induces switched-memory B cells to express RANKL, a process further enhanced by (c) interferon gamma (IFN-γ). In this condition B cells express high levels of CXCR3, then facilitating their recruitment into the inflammatory lesions such as the synovium of patients with RA. Notably, CXCL10, a ligand for CXCR3, further enhances the potential of B cells to produce RANKL. Together with their production of tumor necrosis factor alpha (TNF-α), RANKL-producing effector memory B cells could thus directly promote osteoclast differentiation and bone destruction. HLA human leukocyte antigen, TCR T-cell receptor, RANK receptor activator of nuclear factor kappa-B, TNFR tumor necrosis factor-α receptor
