Abstract
The carotid body is a multi-modal sensor and it has been debated if it senses low glucose. We have hypothesized that the carotid body is modified by some metabolic factors other than glucose and contributes to whole body glucose metabolism. This study examined the roles of insulin, leptin and transient receptor potential (TRP) channels on carotid sinus nerve (CSN) chemoreceptor discharge. In agreement with other studies, CSN activity was not modified by low glucose. Insulin did not affect the CSN hypoxic response. Leptin significantly augmented the CSN response to hypoxia and nonspecific Trp channel blockers (SKF96365, 2-APB) reversed the effect of leptin. Gene expression analysis showed high expression of Trpm3, 6, and 7 channels in the carotid body and petrosal ganglion. The results suggest that the adult mouse carotid body does not sense glucose levels directly. The carotid body may contribute to neural control of glucose metabolism via leptin receptor-mediated TRP channel activation.
Keywords: CSN activity, Glucose, Insulin, Leptin, TRP channel
17.1 Introduction
It is well established that the carotid body plays a key role in physiological responses to hypoxia and hypercapnea in many species and different preparations. Further, function of the carotid body is modified by other physiological stimuli (Gonzalez et al. 1994). A pioneer work by Alvarez-Buylla and Alvarez-Buylla (1988) has opened the debates if the carotid body senses glucose concentration or regulates glucose metabolism. Regarding glucose sensing, conflicting data have been presented and consensus has not been reached. In general, in vitro culture preparations showed glomus cell’s sensitivity to low glucose. For example, catecholamine release from cultured carotid body slices increased in response to low glucose (Pardal and López-Barneo 2002). Afferent neural discharge in co-culture preparation of petrosal neurons and glomus cells increased during low glucose superfusion (Zhang et al. 2007). On the other hand, glucose sensitivity of the carotid body has not been consistently shown in acute preparations. Chemoreceptor discharge was not increased with low glucose (Bin-Jaliah et al. 2004; Conde et al. 2007), although the effect of cyanide on chemoreceptor discharge was enhanced by low glucose (Alvarez-Buylla and Alvarez-Buylla 1988). Moreover, phenotypic changes of glomus cells during culture have been suggested (Gallego-Martin et al. 2012). Nevertheless, several experimental data suggest that the carotid body is involved in whole body glucose metabolism: experimentally induced perturbation of glucose metabolism was blocked by carotid body afferent denervation (Alvarez-Buylla and Alvarez-Buylla 1988; Koyama et al. 2000; Shin et al. 2014; Wehrwein et al. 2010). Hence, we have hypothesized that the carotid body is modified by some metabolic factors and contributes to whole body glucose metabolism. In this study we focused the roles of insulin, leptin and Trp channels on CSN chemoreceptor discharge.
17.2 Methods
Mice (DBA/2 J, C57BL/6 J) were purchased from Jackson Laboratory, housed and bred in the animal facility of the Johns Hopkins Bloomberg School of Public Health. The experimental protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University.
17.2.1 Measurements of CSN Activity
For assessing CSN chemoreceptor activity, the carotid body bifurcation with CSN was obtained from a deeply anesthetized mouse at ~4–6 weeks of age. The tissue was continuously superfused with Krebs solution (in mM: 120 NaCl, 4.7 KCl, 1.2, CaCl2 1.8 mM, Mg SO4·7H2 O, 1.2 Na2HPO4, 22 NaHCO3, and 11.1 Glucose) bubbled with 95 % O2/5 % CO2 or 95 % N2/5 % CO2 (hypoxic challenge) at 35–37 °C. Extra tissues were removed to expose the carotid body and the CSN. The central cut end of CSN was sucked in a glass suction electrode. Signals were collected and analyzed with an AcqKnowledge software (BioPac Systems, Inc.). CSN activity was expressed as impulses/s. At the end of each experiment the nerve was dislodged from the electrode, the electrode was refilled with Krebs solution and the activity at this point was considered as the noise level and subtracted from each measurement. Statistical analysis was performed with GraphPad Prism 4 using repeated one-way ANOVA. P value less than 0.05 was considered significant.
17.2.2 Gene Expression Analysis
Carotid bodies, petrosal ganglia, superior cervical ganglia (SCG), and brainstem were harvested from deeply anesthetized mice in TRIzol solution (Invitrogen), and frozen at −80 °C until use. Total RNA was isolated and reverse transcribed to cDNA with iScript Reverse Transcriptase (BIO- RAD, Hercules, CA). mRNA levels of the genes were quantified by SYBR Green-based real-time PCR using SsoAdvanced Universal SYBR® Green Supermix (BIO-RAD). The 2-ΔΔCt method was used to calculate the relative expression level of transcripts normalized to Rpl19.
17.3 Results
17.3.1 CSN Chemoreceptor Activity
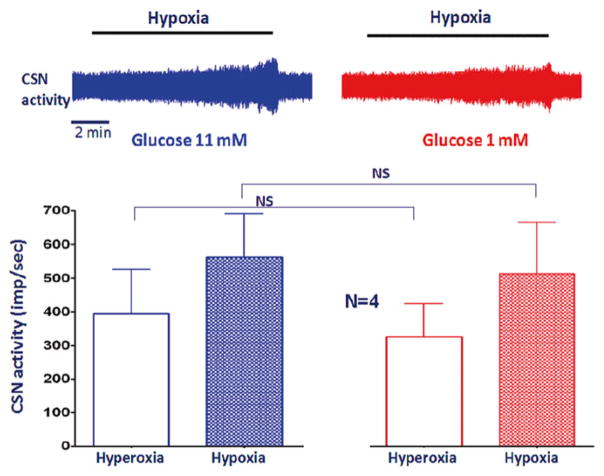
When Krebs was switched to hyperoxia to hypoxia, the chamber PO2 gradually decreased. The nadir was ~40 mmHg. CSN activity in mice increased along with the decrease in PO2 (Fig. 17.1, left). After the basal hypoxic response was recorded, the superfusate was switched to low glucose (1 mM; substituted with 10 mM sucrose) for 5 min, which did not change the CSN activity. The CSN response to hypoxia during low glucose was not enhanced compared to that of high glucose (11 mM). The results agreed with other studies in acute CSN recording (Bin-Jaliah et al. 2004; Conde et al. 2007),
Fig. 17.1.
Hypoxia significantly increased CSN activity. Low glucose did not significantly influence the basal activity or the carotid body response to hypoxia
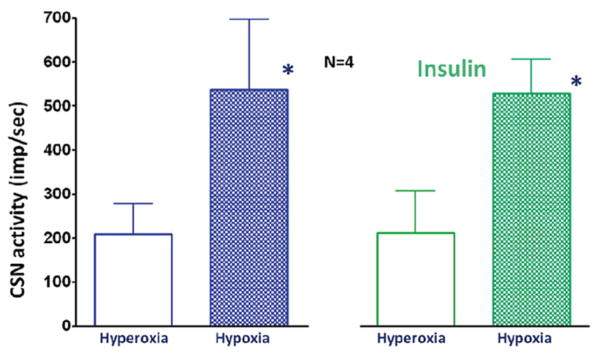
Insulin is a key hormone in whole body glucose metabolism. Further, insulin was used to clamp blood glucose levels for studying the role of the carotid body in glucose regulation (Koyama et al. 2000; Wehrwein et al. 2010). Here, we examined if insulin (5 μg/mL) influenced CSN response to hypoxia. The data show that insulin did not have significant influence on the carotid chemoreceptor hypoxic response (Fig. 17.2).
Fig. 17.2.
Hypoxia significantly increased CSN activity, but insulin did not influence the basal activity nor the carotid body response to hypoxia
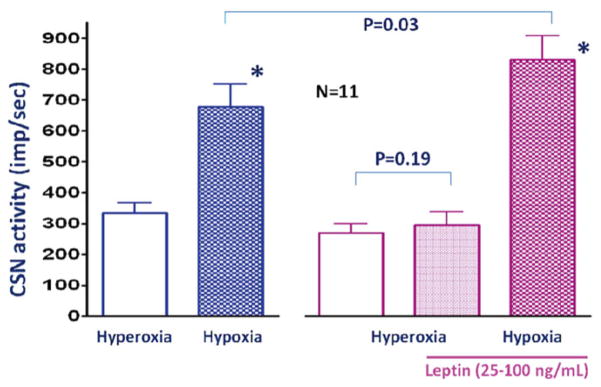
In a previous study we found that leptin deficiency mice have blunted hypoxic ventilatory response (Tankersley et al. 2013). The presence of leptin receptors in the rat and human carotid body have been shown by Porzionato et al. (2011). In agreement with their work, our data showed high level of leptin receptor gene expression in the mouse carotid body (Balbir et al. 2007). Physiological roles of leptin and its receptors in carotid body function are not known. It is possible that leptin has a stimulatory effect on the carotid body. After obtaining the baseline hypoxic response, leptin (25–100 ng/mL) was added to the Krebs. After 5 min perfusion with leptin, hypoxic challenge was repeated. Leptin significantly enhanced the hypoxic chemoreceptor response (Fig. 17.3).
Fig. 17.3.
Leptin perfusion for 5 min did not change CSN activity during hyperoxia. However hypoxic response was significantly enhanced
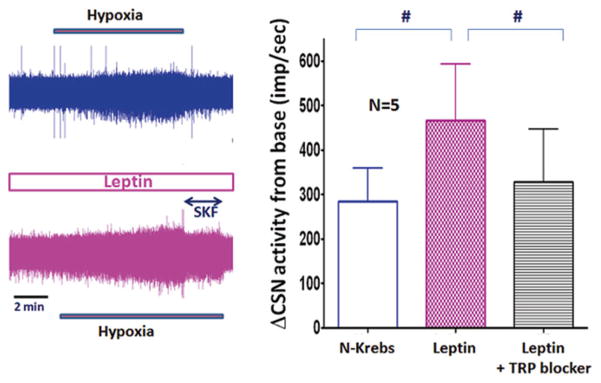
It has been shown that leptin excites proopiomelatonin neurons via activation of TrpC channels (Qiu et al. 2010). Therefore, we further examined if the effect of leptin on the hypoxic response of CSN is mediated via Trp channels. During hypoxic challenge with leptin, a TRP channel blocker (SKF96365 10 μM or 2-APB 10 μM) was added to the superfusate. The results show that TRP channel blockers inhibited the enhanced hypoxic response mediated by leptin (Fig. 17.4).
Fig. 17.4.
The raw traces of CSN activity (top, control; bottom, leptin) show leptin enhanced hypoxic response and non-specific TRP channel blocker (SKF) dampened the activity. In the summary graph (right) the data with SKF and 2-APB were pooled
17.3.2 Gene Expression of TRP Channels
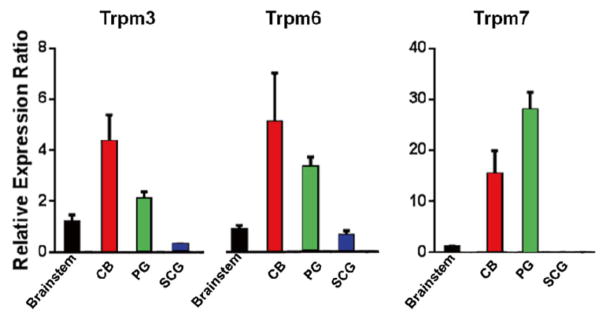
TRP channels are a family of non-selective cation channels. At least 33 subunit genes are encoded and the channels are divided into 7 subfamilies. Among them we examined the mRNA levels of 9 genes from 3 subfamilies (TrpC1, TrpC5, TrpC6, Trpm2, Trpm3, Trpm6, Trpm7, Trpv1, and Trpv2). Compared to the brainstem or the superior cervical ganglia, the carotid body and petrosal ganglia expressed more Trpm type channels (3, 6 and 7; Fig. 17.5). The expression of other channels examined was comparable among tissues (data not presented).
Fig. 17.5.
mRNA levels of Trpm3, Trpm6 and Trpm7 genes in the brainstem, carotid body (CB), Petrosal ganglion (PG) and superior cervical ganglion (SCG). N = 4, error bars: SD
17.4 Discussion
The carotid body is a multi-modal sensor. Several recent studies suggest that it senses low glucose and/or participate in regulating metabolism (by Alvarez-Buylla and Alvarez-Buylla 1988; Koyama et al. 2000; Pardal and López-Barneo 2002; Shin et al. 2014; Wehrwein et al. 2010; Zhang et al. 2007). Considering the well established sensitivity to O2 and CO2, and the location of the carotid body, it is tempting to assume that the carotid body is monitoring metabolic states in the blood that goes to the brain. However to prove that the carotid body is a glucose or metabolic sensor, we need to consider several critical conditions (e.g., sensitivity, low threshold, increase of output etc.). In our model glucose does not influence the carotid chemosensory activity. This result is consistent with other acute studies (Bin-Jaliah et al. 2004; Conde et al. 2007). Although cultured models show glucose sensitivity of glomus cells (Pardal and López-Barneo 2002; Zhang et al. 2007), phenotypic changes in glucose sensitivity during culture has been indicated (Gallego-Martin et al. 2012). Collectively, it seems that the carotid body, if not cultured, does not sense glucose levels directly.
It appears, however, that the carotid body plays a unique role in regulation of whole body glucose metabolism. Stimulation of the carotid body by cyanide increased blood glucose concentration and hepatic glucose output which were abolished by carotid sinus nerve denervation (Alvarez-Buylla and Alvarez-Buylla 1988). Experimental hypoglycemia released several metabolic hormones and enhanced endogenous glucose output, but the carotid body denervation or hyperoxia attenuated these responses (Koyama et al. 2000; Wehrwein et al. 2010). Recently we found that the carotid sinus denervation prevented intermittent hypoxia-induced hyperglycemia (Shin et al. 2014). Thus, the carotid body may participate in the pathogenesis of metabolic diseases.
The current study, together with our previous data showing high expression of leptin receptors in the mouse carotid body (Balbir et al. 2007), suggests that leptin activates leptin receptors in the carotid body and/or nerve endings and enhances CSN response to hypoxia. The results agree with our previous work, showing that leptin deficiency mice have blunted hypoxic ventilatory response (Tankersley et al. 2013). Furthermore, leptin-induced enhancement of hypoxic chemoreceptor response was blocked by TRP channel blockers. Gene expression analysis showed uniquely high expression of Trpm 3, 6, and 7 channels in the carotid body and petrosal ganglion. Because of a lack of specific blockers for these channels, their physiological role in carotid body hypoxic response is not clear yet. Future studies are required to clarify the contribution of leptin and TRP channels to carotid body function. In conclusion, the carotid body may contribute to neural control of glucose metabolism via leptin receptor-mediated TRP channel activation.
Acknowledgments
This work was funded, in part, by HL081345, ES016817, HL080105, and AHA 12POST118200001.
Contributor Information
M. Shirahata, Email: mshiraha@jhmi.edu, Department of Environmental Health Sciences, Johns Hopkins University, Baltimore, MD, USA
W.-Y. Tang, Department of Environmental Health Sciences, Johns Hopkins University, Baltimore, MD, USA
M.-K. Shin, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
V.Y. Polotsky, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
References
- Alvarez-Buylla R, Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988;72:347–360. doi: 10.1016/0034-5687(88)90093-x. [DOI] [PubMed] [Google Scholar]
- Balbir A, Lee H, Okumura M, Biswal S, Fitzgerald RS, Shirahata M. A search for genes that may confer divergent morphology and function in the carotid body between two strains of mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L704–L715. doi: 10.1152/ajplung.00383.2006. [DOI] [PubMed] [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556:255–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Gonzalez C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol. 2007;585:721–730. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Martin T, Fernandez-Martinez S, Rigual R, Obeso A, Gonzalez C. Effects of low glucose on carotid body chemoreceptor cell activity studied in cultures of intact organs and in dissociated cells. Am J Physiol Cell Physiol. 2012;302:C1128–C1140. doi: 10.1152/ajpcell.00196.2011. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Rucinski A, Macchi V, Stecco C, Castagliuoloc I, Malendowicz LK, De Caro R. Expression of leptin and leptin receptor isoforms in the rat and human carotid body. Brain Res. 2011;1385:56–67. doi: 10.1016/j.brainres.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites POMC neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Yao Q, Jun JC, Bevans-Fonti S, Yoo DY, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M, Polotsky VY. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol. 2014;117:765–776. doi: 10.1152/japplphysiol.01133.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankersley CG, Tang W-Y, Wang X, Pardo C, Bierman A, Shirahata M. Effect of leptin on hypoxic ventilator regulation. ATS international conference; 2013. p. A5763. [Google Scholar]
- Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol. 2010;588:4593–4601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Buttigieg J, Nurse CA. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol. 2007;578:735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]