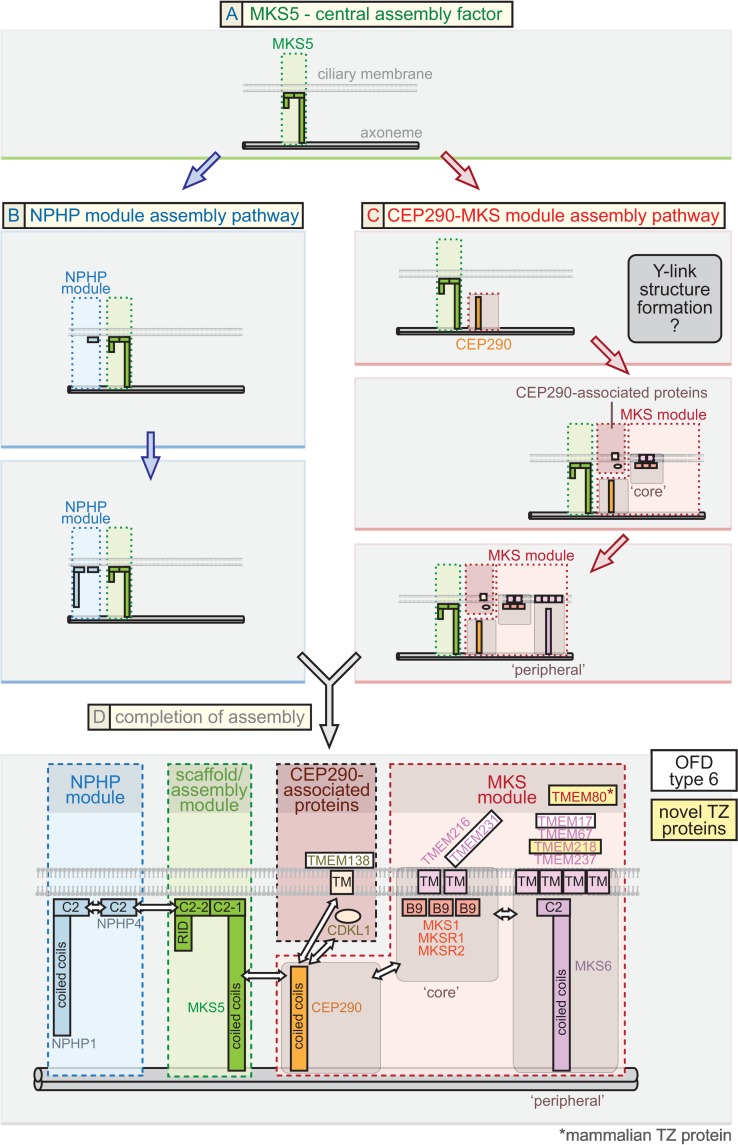
Fig 7. Proposed model for ciliary transition zone assembly.
This model for the assembly of proteins at the TZ is based mostly on C. elegans data but is largely consistent with findings from other organisms, including mammals, Chlamydomonas, and Drosophila. (A) MKS5: central assembly factor. C. elegans MKS-5 (mammalian Rprgrip1L/Rpgrip1 orthologue) is essential for the assembly of all known TZ proteins (including CEP-290) and, therefore, may be the first component to establish the TZ. MKS-5 directs two genetically separable (distinct) assembly pathways, involving NPHP module proteins (blue arrows) as well as CEP-290, CEP-290-associated proteins, and MKS module proteins (red arrows). (B) NPHP module assembly pathway. Results from this study, other C. elegans findings, and mammalian work suggest that NPHP module proteins function together with MKS-5. We propose that the NPHP module, which can assemble at the TZ in the absence of CEP-290 (red pathway), assembles either before or in conjunction with the CEP-290-dependent pathway. NPHP-1 requires NPHP-4 for assembly at the TZ and is, therefore, positioned “downstream” in the pathway. (C) CEP290-MKS module assembly pathway. CEP-290 depends on MKS-5 for its TZ localisation and is, therefore, shown to assemble “downstream” in the pathway. Established MKS module proteins, including “core” components, require CEP-290 for TZ localization and are shown to assemble “downstream.” Another subset of TZ proteins (CDKL-1, TMEM-138) depends on CEP-290, but not MKS module proteins, for TZ localization (termed “CEP290-associated proteins”); their assembly is tentatively shown to occur simultaneously with the “core” MKS module components. TZ ultrastructure (Y-links) can be observed when “core” MKS or NPHP module proteins are disrupted, but not when CEP-290 is removed, and so we tentatively assign the formation of the Y-links at the CEP-290 assembly stage. More “peripheral” MKS module components require “core” MKS module components for TZ localisation and, hence, are proposed to assemble “downstream” of the core components. CEP-290, CEP-290-associated proteins, and MKS module (“core” and “peripheral”) proteins are shown being incorporated at distinct steps of an assembly process, but the possibility of pre-assembly exists; for example, “core” MKS module proteins might pre-assemble prior to incorporation into the TZ, and “peripheral” proteins might interact with the “core” proteins prior to the assembly stage shown. (D) Completion of TZ assembly and link to the ciliopathy OFD6. MKS-5, the NPHP module, and the CEP-290-MKS module all combine to yield the complete TZ. A detailed spatial/topological/hierarchical map of TZ proteins, relative to the axoneme (double cylinders at bottom) and ciliary membrane (bilayer shown), is suggested. Pertinent domains shown in the TZ proteins include membrane-associated C2 and related B9 domains, transmembrane (TMEM) domains, and coiled coils. Major connections (potentially direct or indirect) between different modules or proteins are depicted with double-headed arrows and based on a combination of data from C. elegans and other organisms. Novel TZ proteins we uncovered in this study (C. elegans TMEM-218 and mammalian Tmem80) and proteins we provide evidence for being associated with OFD6 (Tmem17, Tmem138, Tmem231), are highlighted in yellow and white boxes, respectively.