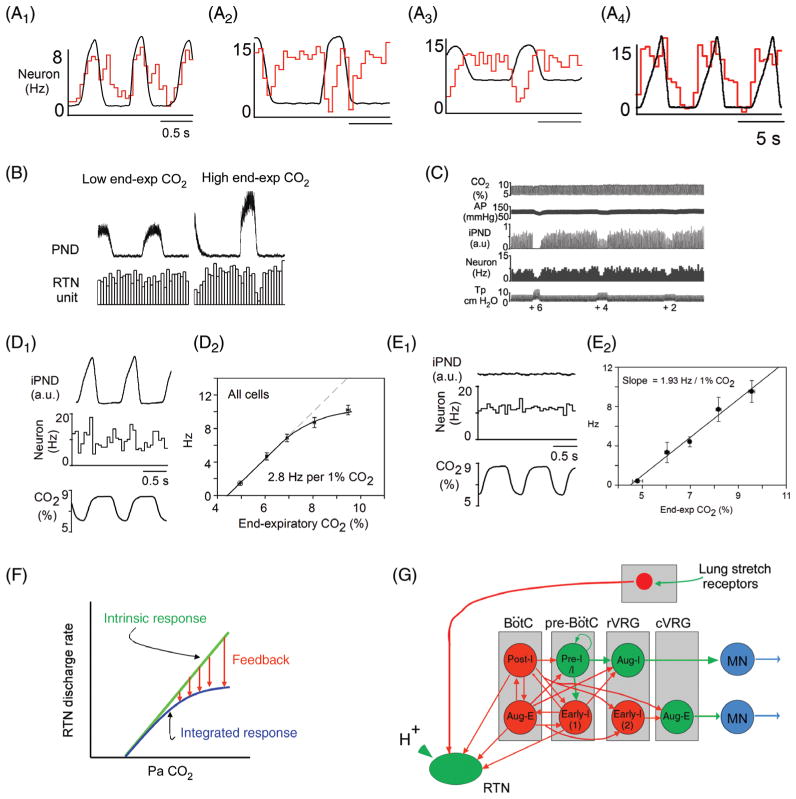
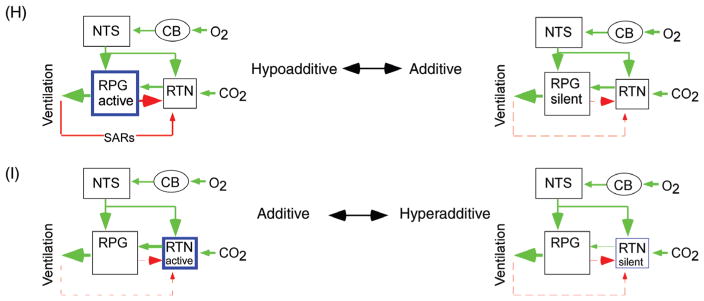
Figure 10.
Feedback regulation of RTN neurons and interaction between central and peripheral respiratory chemoreflexes. (A1–4) Four examples of RTN neurons showing different types of respiratory modulation which can be interpreted as post-I and E-aug inhibition in cell 1, early-I and post-I inhibition in cell 2, early-I only in cell 3, and early-I, post-I and E-AUG in cell 4 [adapted, with permission, from (158)]. (B) Typical RTN neuron devoid of respiratory modulation at low end-expiratory CO2. This cell exhibits the early-I/post-I pattern when FiCO2 is increased (unpublished example from P. Guyenet). C, single RTN neuron inhibited by lung-inflation [Tp, tracheal pressure, iPND integrated rectified phrenic nerve discharge; adapted, with permission, from (295)]. (D1) Single RTN neuron recorded in a vagally intact anesthetized rat showing the complex respiratory modulation of the cell (integrated rate histogram and rectified PND triggered on expiratory CO2 shown as bottom trace). (D2) Average steady-state response of RTN neurons to end-expiratory CO2 in the same preparation as D1 [D1–D2 adapted, with permission, from (158)]. (E1 and E2) Similar recordings after i.c.v. administration of the glutamatergic antagonist kynurenic acid. (E1) PND and the respiratory modulation of the RTN neuron was abolished by kynurenic acid. (E2) The relationship between the discharge rate of RTN neurons and end-expiratory CO2 became linear after kynurenic acid administration [adapted, with permission, from (158)]. (F) Interpretation of the results shown in A–E: the response of RTN neurons to CO2 is saturable because of the existence of inhibitory feedback from lung stretch receptors and from the RPG. G, schematic wiring diagram based on A–E. (H) Hypothetical scenario in which the RPG is unusually active or excitable (left). In such a case, respiratory feedback to the RTN would minimize the contribution of this nucleus to ventilation causing hypoadditivity between the peripheral and central chemoreflexes. On the other hand, additivity would result from a situation in which the RPG is moderately excitable (right panel). (I) Possible hyperadditivity scenario. RTN hyperpolarization at rest (right panel) could result in hyperadditivity between the central and peripheral chemoreflexes. In this configuration, moderate hypercapnia alone would produce a minimal stimulation of breathing because RTN depolarization would be largely subthreshold. Carotid body stimulation would depolarize RTN neurons above their discharge threshold causing them to respond vigorously to the previously ineffective hypercapnic stimulus, hence the apparent hyperadditivity of the reflexes.