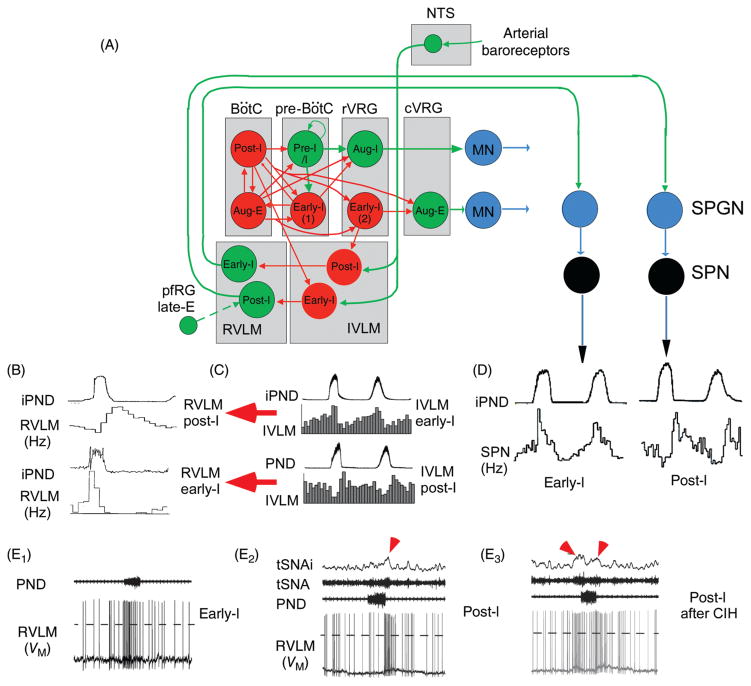
Figure 3.
Regulation of the sympathetic vasomotor outflow by the respiratory pattern generator. (A) Plausible circuitry responsible for coupling respiration with the sympathetic nerve activity to the cardiovascular system. Sympathetic ganglionic neurons (SPNs) display stereotyped patterns of respiratory modulation in deafferented preparations (vagotomized, barodenervated). Two of the most commonly found patterns in rats (early-I and post-I) are depicted in D [top traces, average integrated phrenic nerve discharge, iPND; lower trace iPND-triggered rate histogram depicting the probability of firing of a single SPN during the central respiratory cycle; redrawn, with permission, after (72)]. These SPN patterns are virtually identical to those of single RVLM presympathetic neurons [shown in B; redrawn, with permission, from (168)]. The IVLM contains GABAergic neurons that are activated by arterial baroreceptor stimulation and inhibit RVLM presympathetic neurons (panel A). The respiratory modulation of these IVLM GABAergic neurons is approximately the mirror image of that of the RVLM presympathetic neurons [C; reprinted from Mandel and Schreihofer (265) with permission from Wiley & Sons] hence the hypothesis that IVLM neurons with early-inspiratory discharge produce post-I modulation in RVLM presympathetic neurons and vice versa (red arrows in C). The depicted inputs from the RPG to IVLM neurons are plausible but speculative. (E) Intracellular recordings of RVLM presympathetic neurons in an arterially perfused midcollicular transected rat preparation [from Moraes et al. (293) with permission]. The early-I (left trace) and the post-I patterns (middle trace) are instantly recognizable. The right panel shows a presympathetic RVLM neuron that exhibits an extra peak of activity during late expiration. The latter recording was made in a rat subjected to chronic intermittent hypoxia which caused late-expiratory activity in an abdominal nerve at rest (not shown) and an additional late-E peak of activity in the thoracic chain (red arrow pointing down and to the right above the thoracic chain SNA trace, tSNA). An excitatory input from pfRG has been tentatively proposed as the source of this late-expiratory activation (illustrated in A).