Abstract
Hypodontia can be defined as the non-formation of one or more teeth during the developmental period. Mutation in several genes related to tooth formation has previously been correlated with cancer. Regarding the ovarian cancer, there are few studies that associate the presence of hypodontia with ovarian cancer. A systematic literature search was performed in PubMed and Scopus. In total, 385 patients were included in this study. Control group was present in 3 out of 4 studies (340 patients). Hypodontia was present in 56 out of 290 patients (incidence of 19.3%). Only in 2 out of 4 studies, the number of missing teeth was mentioned (47 teeth), while the majority of them were either maxillary second premolars or maxillary lateral incisors. Unilateral distribution of the missing teeth was present in 28 out of 46 patients, while bilateral distribution of the missing teeth was present in 18 out of 46 patients. The presence of ovarian cancer in the family medical history occurred in 12 out of 33 patients. Only 1 out of 4 studies examined the presence of genes with mutations in the included patients. Based on our findings, the lack of clinical studies was the principal obstacle to clarify the possible predictive value of hypodontia in the early prediction of patients with higher risk of ovarian cancer.
Keywords: Hypodontia, ovarian cancer, EDA gene, AXIN2 gene, WNT10A gene, BRCA gene
Introduction
Hypodontia or selective tooth agenesis can be defined the non-formation of one or more teeth (<6 teeth) during the developmental period combined with variations in size, shape, and eruption time (1). The worldwide prevalence ranges between 2.6% and 11.3% (2, 3). Women are affected more than males at a ratio of 3:2. Hypodontia may be presented either as part of a clinical syndrome or as a non-syndromic form, the latter being more frequent. Both genetic and environmental explanations for hypodontia have been reported (1). More than 300 genes are involved in odontogenesis (4). Mutation in several genes related to tooth formation has previously been correlated with cancer (5–7). Regarding the ovarian cancer, there are few studies that link the presence of hypodontia with ovarian cancer. The purpose of our study is to review the available literature on the correlation of hypodontia and ovarian cancer.
Methods
Data sources
A systematic search was performed in PubMed (May 1, 2015) and Scopus (May 1, 2015) to retrieve the included studies. The applied search strategy, in all the searched databases, included the combination of the key words: hypodontia and ovarian cancer. For additional studies, the references of the included articles have also been hand-searched.
Study selection criteria
All the studies that reported data on the presence of hypodontia and ovarian cancer were included in this review. Abstracts in scientific conferences, animal studies, editorials, letters to the editor, review articles, and studies published in languages other than English, German, Greek, French, Italian, and Spanish were excluded from this review. The retrieved data from each of the included studies were focused on the publication type, the number of patients included in each study, the number of patients included in the control group, the incidence of hypodontia, the number of missing teeth, the type of missing teeth, the distribution of hypodontia, the presence of ovarian cancer in family medical history, and the presence of isolated genes correlated to ovarian cancer.
Results
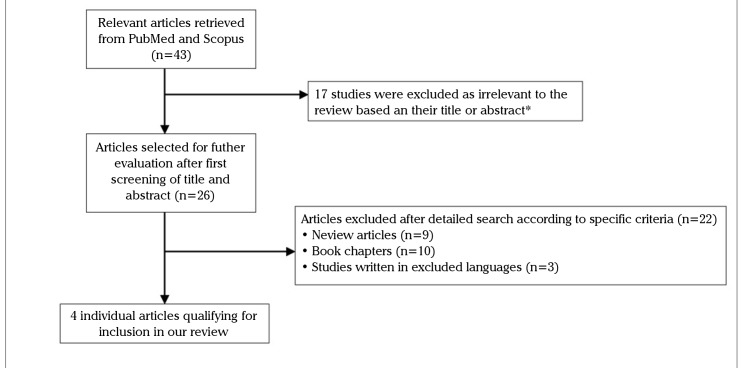
The performed search in PubMed and Scopus revealed a total of 43 and 39 search results, respectively, among which 4 studies (4 case series) were identified as eligible for inclusion in this review, according to the inclusion criteria (8–11). No additional studies were identified by searching the references of the included studies. The selected studies for inclusion are presented in detail in Figure 1 (flow diagram).
Figure 1.
Flow diagram of the selection process of the included articles in the review
*The majority of studies were found in both databases
The principal characteristics of the included studies in our review (publication type, number of patients, control group, incidence of hypodontia, number of missing teeth, type of missing teeth, distribution of hypodontia, presence of ovarian cancer in family medical history, and isolated genes) are presented in Table 1. In total, 385 patients were included in this study. Control group was present in 3 out of 4 studies (340 patients). Hypodontia was present in 56 out of 290 patients (19.3%). Only in 2 out of 4 studies, the number of missing teeth was mentioned (47 teeth), and the majority of them were either maxillary second premolars or maxillary lateral incisors. Unilateral distribution of the missing teeth was present in 28 out of 46 patients, while bilateral distribution of the missing teeth was present in 18 out of 46 patients. The presence of ovarian cancer in the family medical history occurred in 12 out of 33 patients. Only 1 out of 4 studies examined the presence of genes with mutations in the included patients. Specifically, breast cancer 1 (BRCA1) gene and wingless-type MMTV integration site family member 10A (WNT10A) gene was present in 7 and in 6 out of 50 patients, respectively.
Table 1.
Studies reporting data regarding the correlation of hypodontia with ovarian cancer
| First author, year, country (Ref) | Publication type | No. of patients | Control group | Incidence of hypodontia (%) | No. of missing teeth | Type of missing teeth (%) | Distribution of hypodontia (%) | Presence of ovarian cancer in family medical history (%) | Isolated genes (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fekonja et al. (8), 2015, Slovenia | retrospective study | 120 | 120 | 23/120 (19.2) | NM | NM | Unilateral: 10/23 (43.5) Bilateral: 13/23 (56.5) |
9/23 (39) | NM |
| Bonds et al. (9)*, 2014, USA | retrospective study | 95 | - | NM | NM | NM | NM | NM | BRCA1: 7/50 (14) EDA: 1/50 (2) WNT10A: 6/50 (12) AXIN2: 1/50 (2) |
| Fekonja et al. (10), 2014, Slovenia | NRCT | 120 | 120 | 23/120 (19.2) | 31 | maxillary SP: 14/31 (45.1) maxillary LI: 10/31 (32.3) mandibular SP: 5/31 (16.1) mandibular CI: 2/31 (6.5) |
Unilateral: 18/23 (78.3) Bilateral: 5/23 (21.7) |
NM | NM |
| Chalothorn et al. (11), 2008, USA | NRCT | 50 | 100 | 10/50 (20) | 16 | maxillary LI, maxillary SP | NM | 3/10 (30) | NM |
USA: United States of America; y.o.: years old; NM: not mentioned; NRCT: non-randomized controlled trial; SP: second premolars; LI: lateral incisors; CI: central incisors; BRCA1: breast cancer 1; EDA: ectodysplasin A; WNT10A: wingless-type MMTV integration site family member 10A; AXIN2: axis inhibition protein 2
Refers to patients included in the Chalothorn et al. (11) study
Discussion
Tooth embryogenesis is controlled by migration of neural crest cells that specialize in formulating specific type teeth. Genetic control during embryogenesis could be related to teeth shape, type, affiliation, and prolapse with the help of homeobox genes such as msh homeobox (MSX) 1 and MSX2 (12). Hypodontia is the most common congenital anomaly of the teeth (13). The data so far suggested that the prevalence is around 3.5–6.5% of the general population; however, it is higher in Africa, where it reaches 13.4% (1). Our review of the current studies revealed an incidence of 19.3% in patients with ovarian cancer.
It is thought to be a multifactorial condition related both to genes as well as environmental effect. In recent studies, it was shown that genes related to hypodontia could also be related to ovarian cancer as women with hypodontia have 8 times higher risk of ovarian cancer (11). Some of the genes that are related to hypodontia are paired box 9 (PAX9), MSX1, and axis inhibition protein 2 (AXIN2) (14, 15). WNT10A is the most commonly mutated gene in hypodontia (16). These genes are also expressed in tumor cells of the female reproductive system. Hypodontia was also associated with positive self-reported family history of cancer and with variants in genes fibroblast growth factor 3 (FGF3), FGF10, and FGFR2 (17). However, BRCA genes show no evidence of involvement in hypodontia, although they are highly associated with ovarian cancer (9). Regarding environmental effect, it is related to early radiation of dental germ, thalidomide, osteomyelitis, or moving the dental germ of the permanent tooth during extraction of milk teeth (18). A family history could be related to hypodontia; however, there is no correlation with maternal health problems during pregnancy (13). Hypodontia is also related to extradermal dysplasia, cleft lip and palate, van der Woude syndrome, and Down syndrome (18).
The teeth that are mainly related to hypodontia are maxillary second premolars or maxillary lateral incisors (10). The lower premolars are usually missing in the Caucasians, while the lower lateral incisors in the Chinese population (10). Our review confirmed that the majority of the teeth are either maxillary second premolars or maxillary lateral incisors. Unilateral distribution of the missing teeth was present in 60.8% of the patients included in the review.
Different parameters are correlated with ovarian cancer such as history of diabetes mellitus or endometriosis (19, 20). The review of the current literature reveals strong correlation between hypodontia and ovarian cancer. Recently, Fekonja et al. (8) performed a subanalysis of their data showing that bilateral ovarian cancers are more common than unilateral in patients with hypodontia, and these patients have a higher incidence of other malignant tumors as well compared to controls. However, Lindor et al. (21) did not find any correlation of colorectal cancer with hypodontia. On the other hand, it was also revealed that there is no correlation of hypodontia with histological ovarian cancer subtype, but a statistically significant difference was found in regard to stage and grade compared to the control group (8).
Our review supports a possible correlation between hypodontia and ovarian cancer, and for this reason, hypodontia could potentially become a risk marker for future ovarian cancer development. A future step could be the earlier referral of such patients for ovarian cancer screening. According to van Nagell et al. (22), if a correlation was found between hypodontia and ovarian cancer in more prospective studies, then semiannual screening should become the standard of care for those women in order to achieve earlier detection.
Before reaching any conclusion, there are several limitations that should be taken into consideration. The limited number of the existing studies and, as a consequence, the small number of the included patients cannot allow us to draw any safe conclusion. However, our review reveals quite a strong correlation between hypodontia and ovarian cancer. Regarding the adopted search strategy, this could be considered as restricted due to the exclusion of abstracts in scientific conferences, review articles, conference papers, letters to the editor, animal studies, and editorials, while the limitation based on the language of the excluded articles could also be considered another weakness of this study.
Conclusion
Based on our findings, we believe that more prospective or retrospective studies should be organized in order to clarify the possible predictive value of hypodontia in the early prediction of patients with higher risk of ovarian cancer.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.I.; Design - C.I., I.D.G.; Supervision - C.I., Resource - C.I., I.D.G.; Materials - C.I., I.D.G.; Data Collection and/or Processing - I.D.G.; Analysis and/or Interpretation - C.I., I.D.G.; Literature Search - I.D.G; Writing - C.I., M.P., I.D.G.; Critical Reviews - C.I., M.P., I.D.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Khalaf K, Miskelly J, Voge E, Macfarlane TV. Prevalence of hypodontia and associated factors: a systematic review and meta-analysis. J Orthod. 2014;41:299–316. doi: 10.1179/1465313314Y.0000000116. http://dx.doi.org/10.1179/1465313314Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 2.Mattheeuws N, Dermaut L, Martens G. Has hypodontia increased in Caucasians during the 20th century? A meta-analysis. Eur J Orthod. 2004;26:99–103. doi: 10.1093/ejo/26.1.99. http://dx.doi.org/10.1093/ejo/26.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Silva Meza R. Radiographic assessment of congenitally missing teeth in orthodontic patients. Int J Paediatr Dent. 2003;13:112–6. doi: 10.1046/j.1365-263x.2003.00436.x. http://dx.doi.org/10.1046/j.1365-263X.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 4.De Coster PJ, Marks LA, Martens LC, Huysseune A. Dental agenesis: genetic and clinical perspectives. J Oral Pathol Med. 2009;38:1–17. doi: 10.1111/j.1600-0714.2008.00699.x. http://dx.doi.org/10.1111/j.1600-0714.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 5.Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–50. doi: 10.1086/386293. http://dx.doi.org/10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid S, Bieber M, Zhang F, Zhang M, He B, Jablons D, et al. Wnt and hedgehog gene pathway expression in serous ovarian cancer. Int J Gynecol Cancer. 2011;21:975–80. doi: 10.1097/IGC.0b013e31821caa6f. http://dx.doi.org/10.1097/IGC.0b013e31821caa6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber JK, Richter T, Kremmer E, Adamski J, Hofler H, Balling R, et al. Progressive loss of PAX9 expression correlates with increasing malignancy of dysplastic and cancerous epithelium of the human oesophagus. J Pathol. 2002;197:293–7. doi: 10.1002/path.1115. http://dx.doi.org/10.1002/path.1115. [DOI] [PubMed] [Google Scholar]
- 8.Fekonja A, Cretnik A, Zerdoner D, Takac I. Hypodontia phenotype in patients with epithelial ovarian cancer. Radiol Oncol. 2015;49:65–70. doi: 10.2478/raon-2014-0034. http://dx.doi.org/10.2478/raon-2014-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonds J, Pollan-White S, Xiang L, Mues G, D’Souza R. Is there a link between ovarian cancer and tooth agenesis? Eur J Med Genet. 2014;57:235–9. doi: 10.1016/j.ejmg.2014.02.013. http://dx.doi.org/10.1016/j.ejmg.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fekonja A, Cretnik A, Takac I. Hypodontia prevalence and pattern in women with epithelial ovarian cancer. Angle Orthod. 2014;84:810–4. doi: 10.2319/112813-876.1. http://dx.doi.org/10.2319/112813-876.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalothorn LA, Beeman CS, Ebersole JL, Kluemper GT, Hicks EP, Kryscio RJ, et al. Hypodontia as a risk marker for epithelial ovarian cancer: a case-controlled study. J Am Dent Assoc. 2008;139:163–9. doi: 10.14219/jada.archive.2008.0132. http://dx.doi.org/10.14219/jada.archive.2008.0132. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Qu HC, Zhang Y. Association of MSX1 and TGF-beta1 genetic polymorphisms with hypodontia: meta-analysis. Genet Mol Res. 2014;13:10007–16. doi: 10.4238/2014.November.28.5. http://dx.doi.org/10.4238/2014.November.28.5. [DOI] [PubMed] [Google Scholar]
- 13.Chosack A, Eidelman E, Cohen T. Hypodontia: a polygenic trait--a family study among Israeli Jews. J Dent Res. 1975;54:16–9. doi: 10.1177/00220345750540011101. http://dx.doi.org/10.1177/00220345750540011101. [DOI] [PubMed] [Google Scholar]
- 14.Mostowska A, Biedziak B, Jagodzinski PP. Axis inhibition protein 2 (AXIN2) polymorphisms may be a risk factor for selective tooth agenesis. J Hum Genet. 2006;51:262–6. doi: 10.1007/s10038-005-0353-6. http://dx.doi.org/10.1007/s10038-005-0353-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Jian F, Chen J, Wang H, Lin Y, Yang Z, et al. Sequence analysis of PAX9, MSX1 and AXIN2 genes in a Chinese oligodontia family. Arch Oral Biol. 2011;56:1027–34. doi: 10.1016/j.archoralbio.2011.03.023. http://dx.doi.org/10.1016/j.archoralbio.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Bohring A, Stamm T, Spaich C, Haase C, Spree K, Hehr U, et al. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet. 2009;85:97–105. doi: 10.1016/j.ajhg.2009.06.001. http://dx.doi.org/10.1016/j.ajhg.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchler EC, Lips A, Tannure PN, Ho B, Costa MC, Granjeiro JM, et al. Tooth agenesis association with self-reported family history of cancer. J Dent Res. 2013;92:149–55. doi: 10.1177/0022034512468750. http://dx.doi.org/10.1177/0022034512468750. [DOI] [PubMed] [Google Scholar]
- 18.Parkin N, Elcock C, Smith RN, Griffin RC, Brook AH. The aetiology of hypodontia: the prevalence, severity and location of hypodontia within families. Arch Oral Biol. 2009;54(Suppl 1):S52–6. doi: 10.1016/j.archoralbio.2008.11.002. http://dx.doi.org/10.1016/j.archoralbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Vrachnis N, Iavazzo C, Iliodromiti Z, Sifakis S, Alexandrou A, Siristatidis C, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet. 2016;293:239–46. doi: 10.1007/s00404-015-3858-z. http://dx.doi.org/10.1007/s00404-015-3858-z. [DOI] [PubMed] [Google Scholar]
- 20.Kondi-Pafiti A, Papakonstantinou E, Iavazzo C, Grigoriadis C, Salakos N, Gregoriou O. Clinicopathological characteristics of ovarian carcinomas associated with endometriosis. Arch Gynecol Obstet. 2012;285:479–83. doi: 10.1007/s00404-011-1957-z. http://dx.doi.org/10.1007/s00404-011-1957-z. [DOI] [PubMed] [Google Scholar]
- 21.Lindor NM, Win AK, Gallinger S, Daftary D, Thibodeau SN, Silva R, et al. Colorectal cancer and self-reported tooth agenesis. Hered Cancer Clin Pract. 2014;12:7. doi: 10.1186/1897-4287-12-7. http://dx.doi.org/10.1186/1897-4287-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Nagell JR, DePriest PD, Ueland FR, DeSimone CP, Cooper AL, McDonald JM, et al. Ovarian cancer screening with annual trans-vaginal sonography: findings of 25,000 women screened. Cancer. 2007;109:1887–96. doi: 10.1002/cncr.22594. http://dx.doi.org/10.1002/cncr.22594. [DOI] [PubMed] [Google Scholar]