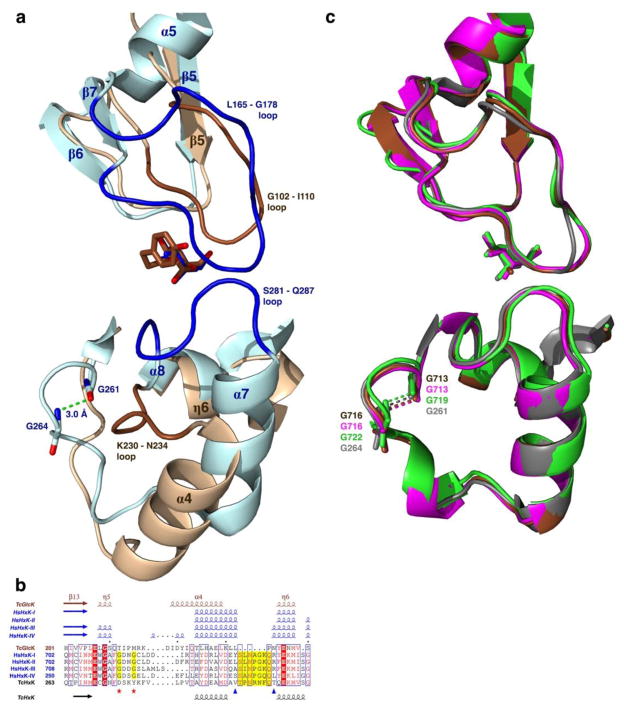
Figure 7.
Primary, secondary, and tertiary structure representations of TcGlcK and HsHxKIV at an outer part of the active site of the monomer. (a) Tertiary structure representations for human hexokinase IV (blue) bound to glucose (PDB entry 4MLE) (54) that is superimposed onto the TcGlcK-HPOP-GlcN complex (brown). (b) Multiple sequence alignment of the TcGlcK H201 – S240 segment by means of the Clustal Omega program (55), where TcGlcK (PDB coordinates from the TcGlcK-DBT-GlcN complex) is aligned with human hexokinase I (PDB entry 4F9O), human hexokinase II (PDB entry 2NZT), human hexokinase III (PDB entry 3HM8), human hexokinase IV (PDB entry 3IDH), and T. cruzi hexokinase (GenBank accession number CAD26835.1). Residues of the HsHxKIV S281 – Q287 loop are highlighted in yellow and are between the two blue triangles. The two red stars indicate the location of the two glycines in the human hexokinases and are highlighted yellow that give rise to the β-turn. All 2° structure elements (α-helices, 310-helices, and β-strands) above the sequence alignment were generated from experimentally determined 3° structures (PDB entries) through the program STRIDE (36, 37) and are color-coded as brown for TcGlcK and blue for the human hexokinases. The 2° structure elements below the sequence alignment (α-helices and β-strands) were generated by prediction from a 1° structure (FASTA entry) through the program PSIPRED (version 3.3) (56) and are color-coded as black for TcHxK. The multiple sequence alignment was created with the program ESPript (version 3.0) (38). (c) Superposition of the A-chains of the human hexokinase isozymes in the closed conformation complexed with glucose in the active site, as follows: hexokinase I (PDB entry 4F9O, brown), hexokinase II (PDB entry 2NZT, magenta), hexokinase III (PDB entry 3HM8, light green), and hexokinase IV (PDB entry 4MLE, grey). The superposition reveals the similarity from the two loops [(L165 – G178); (S281 – Q287)] of hexokinase IV as well as the β-turn, when compared to the other 3 human hexokinase isozymes I–III.