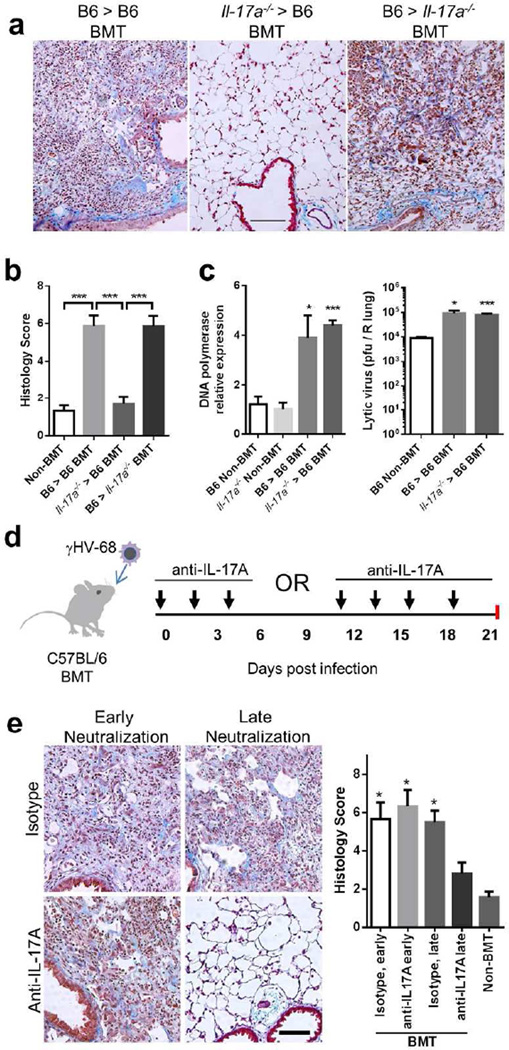
Figure 4. Bone marrow-derived IL-17A is required for development of pneumonitis and fibrosis in γHV-68 infected BMT mice.
(a) Representative Masson’s trichrome staining of lung sections from WT and Il-17a−/− BMT chimeric mice infected with γHV-68 virus at 21 dpi. The blue staining represents deposition of collagen. Same magnification for all images (bar = 100 µm). (b) The average histology scores of lung sections from γHV-68 infected non-BMT or BMT mice as described in (a) at 21 dpi (mean + SEM, n = 5). (c) Lytic replication of γHV-68 in non-BMT WT or Il-17a−/−, and in WT recipients of WT grafts or Il-17a−/− grafts at 7 dpi. Left, mRNA abundance of viral DNA polymerase as measured by RT-PCR (mean + SEM, n = 5); right, the number of lytic virus per right lung was directly measured by plaque assay (mean + SEM, n = 5). Each group was compared to WT non-BMT mice. (d) A scheme of γHV-68 infection and subsequent administration of IL-17A neutralization antibodies in WT BMT mice. (e) The requirement of IL-17A for development of pneumonitis and fibrosis in BMT mice during late stages. Left, representative Masson’s trichrome staining of lung sections from antibody treated BMT mice at 21 dpi. BMT mice received anti-IL-17A antibodies or isotype treatment at early or late time points as shown in (d). The blue staining represents deposition of collagen. Right, average histology scores of lung sections from γHV-68 infected non-BMT or BMT mice treated with anti-IL-17A antibodies or isotype (mean + SEM, n = 4). Each group was compared to BMT mice treated with anti-IL-17A antibodies at late time points. * P < 0.05 and *** P <0.001. Similar results were seen in 2 additional experiments (a, b) or one additional experiment (c, e).