Figure 3.
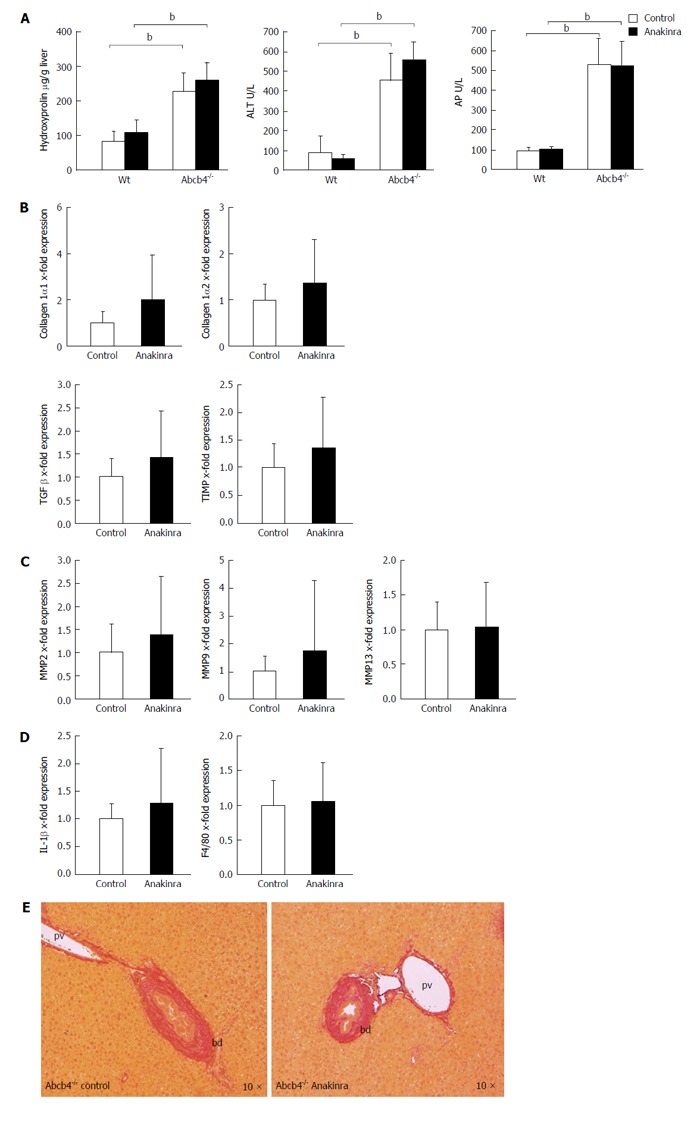
Anakinra does not reveal therapeutic effects on liver injury in ATP-binding cassette transporter b4-/- mice. Eight-week-old female Abcb4-/- animals were treated with daily intraperitoneal injections of saline (control) as vehicle or Anakinra (1 mg/kg body-weight) for 4 wk. A: The levels of hepatic hydroxyproline, alanine aminotransferase (ALT), and alkaline phosphatase (AP) were determined as described previously (n = 8-11, bP < 0.01; Mann-Whitney U-test). Gene expression was assessed via quantitative real-time PCR using glyceraldehyde-3-phosphate dehydrogenase as the housekeeping gene. Data were normalised against the means of the controls (saline); B: No alterations in the profibrotic genes were found [n = 10-11; not significant (n.s.); Mann-Whitney U-test]; C: No alterations in the antifibrotic genes were found (n = 10-11; n.s.; Mann-Whitney U-test); D: Anakinra treatment did not result in a significant change in the mRNA expression of the pro-inflammatory gene IL-1β or F4/80 (n = 10-11, n.s., Mann-Whitney U-test); E: The Sirius-red staining illustrates the absence of antifibrotic effects (original magnification 10 ×). bd: Bile duct; pv: Portal-vein; PCR: Polymerase chain reaction; TIMP: Tissue inhibitor of metalloproteinase; Abcb4-/-: ATP-binding cassette transporter b4-/-; IL: Interleukin; MMP: Matrix metalloproteinase; TGF: Transforming growth factor.
