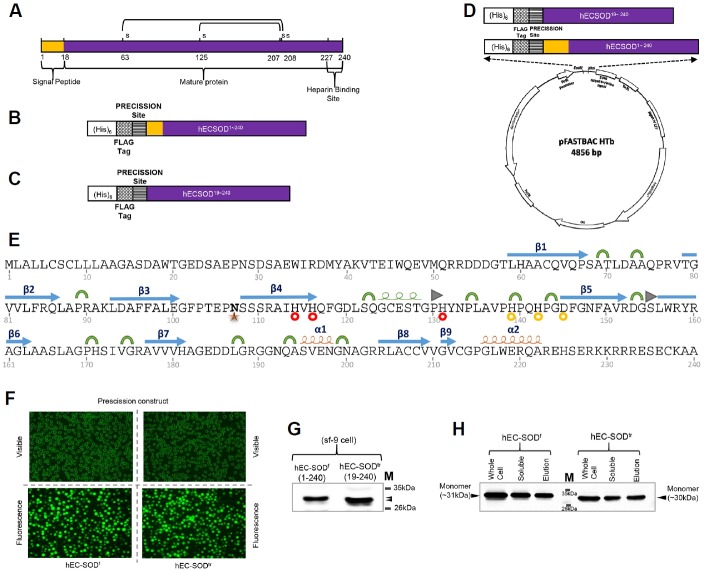
Fig. 1.
Construct design, expression, and purification of hEC-SOD protein from Sf9 insect cells. (A) Schematic illustration of hEC-SOD. The protein consists of an N-terminal signal peptide, mature protein, and a C-terminal heparin-binding site. The design of the construct of (B) hEC-SODf and (C) hEC-SODtr, with an N-terminal (His)6, a FLAG-tag and Precession enzyme site. (D) Map of the hEC-SOD expression vector, pFastBac HTB, containing the pH promoter, a pBR322 origin, and Ampicillin and Gentamycin resistance genes. A full length (residues 1–240) and truncated/mature form (residues 19–240) of hEC-SOD were first amplified by PCR, using primers encoding a FLAG-tag (DYKDDDDK) and PreScission cleavage enzyme recognition sequence (LEVLFQG) at the N-terminus, as well as EcoRI and XhoI restriction enzyme sites. (E) Secondary structure showing the glycosylation site and Cu/Zn binding site of hEC-SOD1–240 based on the crystal structure. PDB code: 2JLP. [
 α-helix,
α-helix,
 310-helix,
310-helix,
 β-strand,
β-strand,
 Turn,
Turn,
 β-bridge,
β-bridge,
 N107 Glycosylation Site,
N107 Glycosylation Site,
 Cu binding site,
Cu binding site,
 Zn binding site]. (F) Transfected recombinant hEC-SOD expressed in Sf9 cells observed at the EGFP-excitation wavelength. (G) Western blot analysis of hEC-SOD expressed in Sf9 cells that had been incubated for 3 days at 27°C, detected using an anti-FLAG antibody. (H) Western analysis of purified hEC-SOD resolved by 12% SDS–PAGE and detected using an anti-FLAG antibody. The size of the expressed hEC-SODf and hEC-SODtr was about 31 and 30 kDa respectively. hEC-SODf: Lane 1, whole cell lysate; lane 2, soluble fraction; and lane 3, purified protein. hEC-SODtr: Lane 5, whole cell lysate; lane 6, soluble fraction; and lane 7, purified protein. Lane 4, molecular marker.
Zn binding site]. (F) Transfected recombinant hEC-SOD expressed in Sf9 cells observed at the EGFP-excitation wavelength. (G) Western blot analysis of hEC-SOD expressed in Sf9 cells that had been incubated for 3 days at 27°C, detected using an anti-FLAG antibody. (H) Western analysis of purified hEC-SOD resolved by 12% SDS–PAGE and detected using an anti-FLAG antibody. The size of the expressed hEC-SODf and hEC-SODtr was about 31 and 30 kDa respectively. hEC-SODf: Lane 1, whole cell lysate; lane 2, soluble fraction; and lane 3, purified protein. hEC-SODtr: Lane 5, whole cell lysate; lane 6, soluble fraction; and lane 7, purified protein. Lane 4, molecular marker.