Fig. 2.
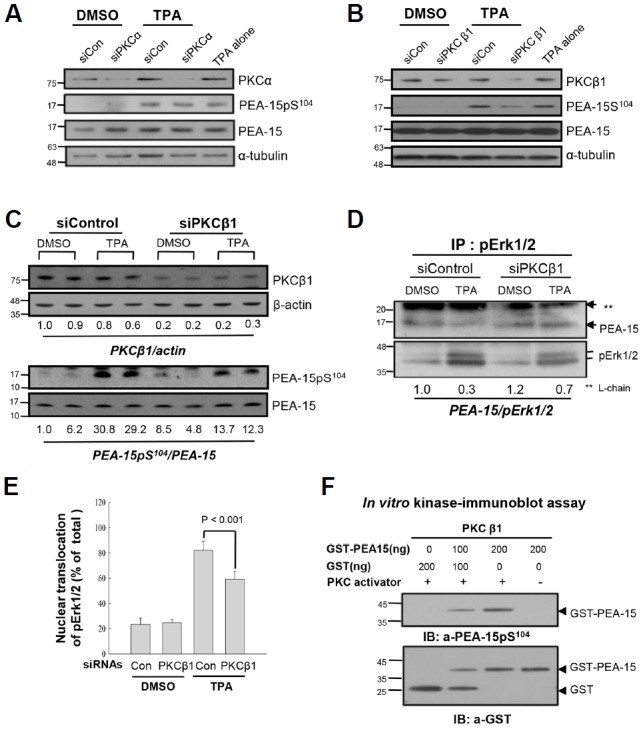
PKCβ1, not PKCα, regulates phosphorylation of PEA-15 at S104 in response to TPA, which dissociates pErk1/2 from PEA-15 in HDF old cells. (A) To explore PKC isozymes which regulate PEA-15pS104, knockdown of PKCα was performed by transfection of senescent HDF cells with siRNAs to PKCα (siPKCα) for 48 h, and the cells were treated with DMSO or TPA for 30 min before IB analysis. The scrambled siRNAs (siCon) and α-tubulin were used as controls for transfection and immunoblot analyses, respectively. Note persistence of PEA-15pS104 after TPA treatment in the siPKCα transfected cells similar to the TPA alone treated cells, indicating not so significant effect of PKCα on the regulation of PEA-15 phosphorylation. (B) To investigate effect of PKCβ1 on the TPA-mediated phosphorylation of PEA-15 at S104, senescent HDF cells were transfected with siRNAs to PKCβ1 (siPKCβ1) for 48 h and then treated with TPA for 30 min. The cell lysates were analyzed by IB with anti-PKCβ1, anti-PEA-15pS104 or anti-PEA-15 antibodies. Note downregulation of PEA-15 phosphorylation after knockdown of PKCβ1 compared with that of the control. siCon and α-tubulin were used as controls for transfection and immunoblot analyses, respectively. (C) To confirm the role of PKCβ1 in the regulation of PEA-15 phosphorylation, degradation of PKCβ1 was manipulated by transfection with siPKCβ1 for 48 h and then changes of PEA-15pS104 by TPA treatment for 30 min were measured based on the amount of PEA-15 expression. PKCβ1 expression was downregulated up to 20–30% than that of the siCon, based on actin expression (PKCβ1/actin; 0.2–0.3 vs.1.0), whereas TPA-induced PEA-15 phosphorylation was reduced more than 50% by knockdown of PKCβ1 (PEA-15pS104/PEA-15 ratio; 12.3–13.7 vs. 30). (D) To evaluate whether knockdown of PKCβ1 regulates interaction of pErk1/2 with PEA-15 or not, HDF old cells transfected with either siCon or siPKCβ1 were treated with TPA for 30 min and then the cell lysates were subjected to IP-IB analysis. TPA treatment significantly reduced interaction of pErk1/2 with PEA-15 (0.3 vs. 1.0), however, it was recovered in part by transfection of siPKCβ1 than that of the siCon (0.7 vs. 0.3). The data strongly suggest in vivo regulation of PEA-15 phosphorylation at S104 residue by PKCβ1 in response to TPA. (E) To explore whether PKCβ1-induced PEA-15pS104 regulates nuclear translocation of pErk1/2 in response to TPA, more than 500 old cells were captured and the cells with pErk1/2 in nuclei were counted under the microscope. Bars represent the means ± SD after 3 independent experiments. TPA-induced nuclear translocation of pErk1/2 was significantly reduced by transfection with siPKCβ1 compared with that of the siControl (p < 0.001), indicating that PKCβ1 is active in the regulation of pErk1/2 translocation to nuclei via PEA-15 phosphorylation at S104 residue. (F) To evaluate activity of PKC isozyme regulating PEA-15pS104, in vitro kinase assay was performed for 30 min at 30°C with GST-PEA-15 as a substrate and PKCβ1 with or without PKC activator. PEA-15 phosphorylation at S104 residue was determined by immunoblot analysis with anti-PEA-15pS104antibody. PKCβ1 activity was increased in the substrate concentration dependent manner in the presence of PKC activator. Hybridization of the reaction mixture with anti-GST antibody showed proteins loaded into each reaction.
