Fig. 7.
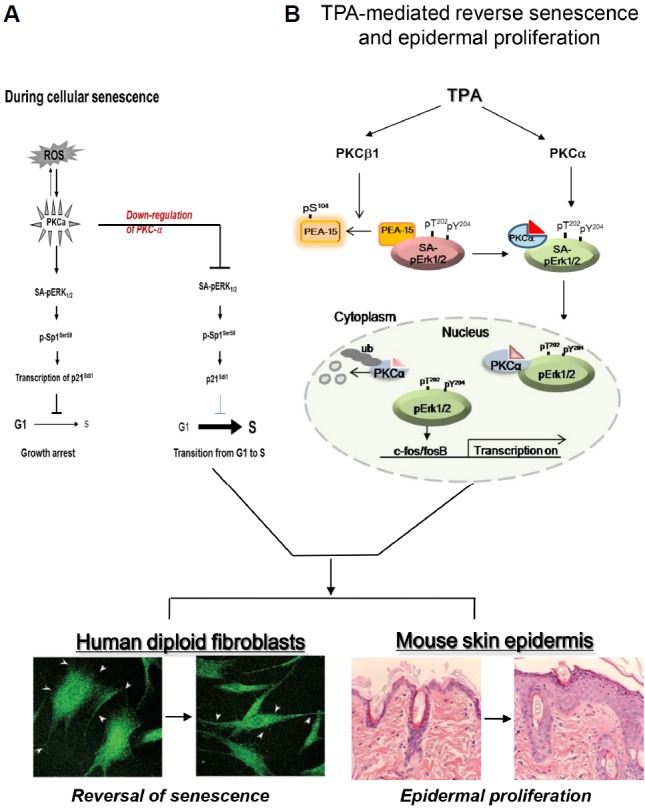
PKCα and PKCβ1 plays differentially in the nuclear translocation of pErk1/2 upon TPA treatment which regulates reversal of senescence phenotypes. (A) During replicative senescence, highly accumulated ROS triggers activation of PKC isozymes without their protein expression, which leads to the induction of p21WAF1 ex-pression through the activations of Erk1/2 and Sp1 transcription factor. However, knockdown of PKCα isoform by transfection with siRNAs reverses senescence program and rather induces cell proliferation after overcome the G1/S arrest. The diagram has been reported by the authors (Kim and Lim, 2009). (B) PKCβ1 activated by TPA regulates in vivo phosphorylation of PEA-15 at S104 residue, which triggers dissociation of SA-pErk1/2 (Senescence Associated-pErk1/2) sequestered in cytoplasm from PEA-15. TPA-stimulated PKCα binds to SA-pErk1/2 released from PEA-15pS104 and then translocated to nuclei of the cells. In the nucleus, pErk1/2 is released from PKCα after its ubiquitination, which triggers a series of gene expression that participates in reversal of the large and flat senescence cells to the actively growing young cells. TPA-induced series of gene expression can be shared by tumor promoting response in skin, which resulted in epidermal proliferation.
