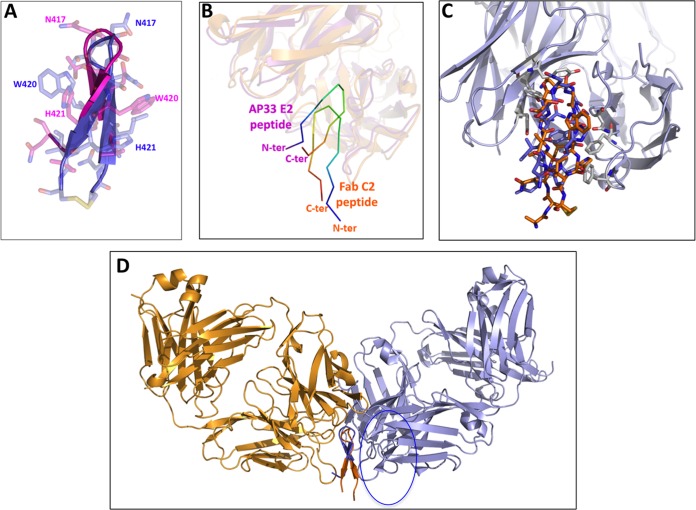
FIG 8.
Comparison of the conformations adopted by epitope 412-422 peptides in complex with Fabs. (A) A structural alignment of peptides bound to AP33 (blue) and to Fab C2 (magenta) shows similar β-hairpin conformations. (B) The crystal structures of Fab C2 (orange; this study) and the Fab of AP33 (purple; PDB accession number 4GAG) are superimposed and faded out. The peptide carbon atoms are ramp colored from the N terminus (blue) to the C terminus (red) through green, to show that the two peptides are bound in opposite orientations relative to the Fab fragment. (C) Superimposition of the cyclic peptide (with the carbon atoms in orange) to the linear peptide in its complex with AP33. (D) The peptide structures in the complexes with Fab C2 (orange) and the Fab of AP33 (purple) are aligned to show how the two antibodies approach the opposite surfaces of the peptide hairpin-like structure.