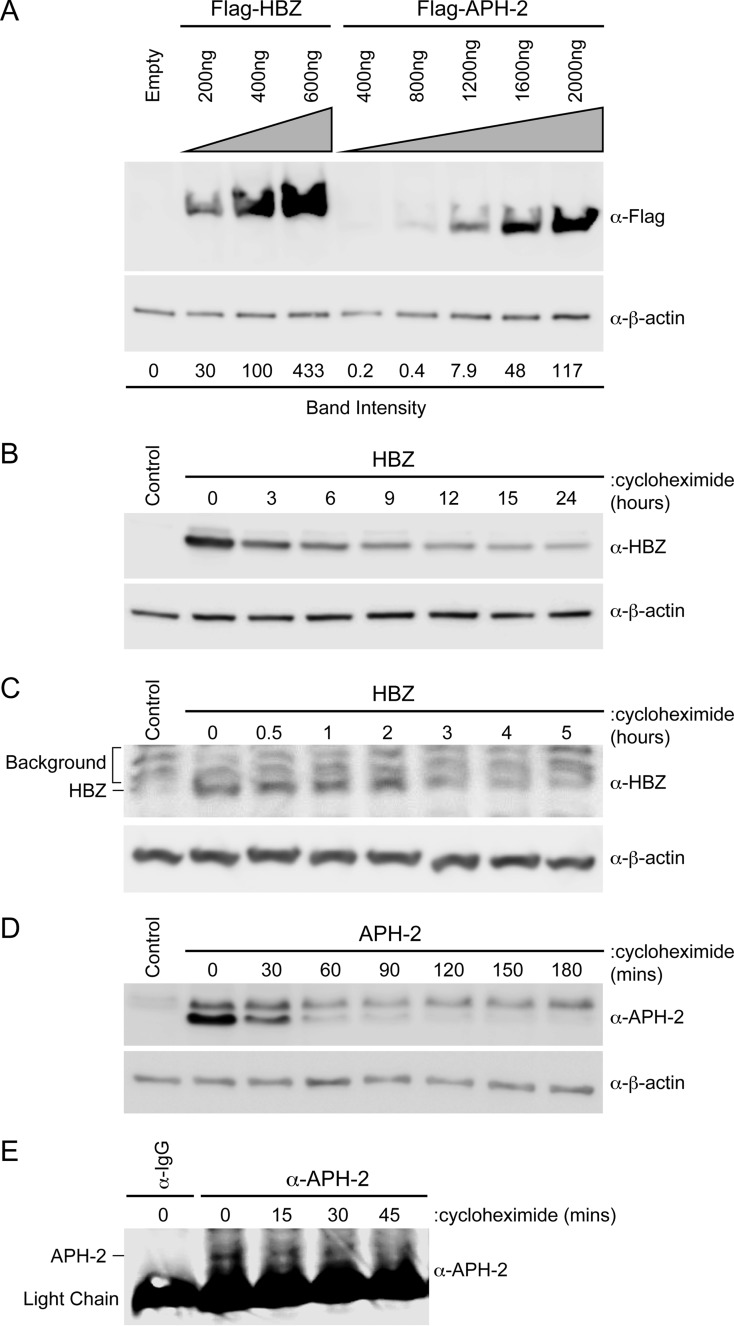
FIG 1.
The APH-2 protein was less stable than the HBZ protein. (A) HEK293T cells were transfected with titrating amounts of FLAG-HBZ, FLAG–APH-2, or the control expression vector (Empty), as indicated. Immunoblot analysis was performed 48 h after transfection to compare the levels of transfected HBZ and APH-2. β-Actin expression was used as a loading control. The amount of FLAG-tagged HBZ or APH-2 relative to the amount of β-actin was measured under each condition. (B and D) HEK293T cells were transfected with 6 μg HBZ (B) or APH-2 (D) expression plasmid in 10-cm dishes. Cells were trypsinized and at 48 h after transfection were replated into 6-well dishes until they reached approximately 80 to 90% confluence. The cells were then treated with 100 μg/ml cycloheximide for the indicated times. Cells were collected by centrifugation, washed in PBS, and lysed using NP-40 lysis buffer. Immunoblot analysis was performed to detect HBZ and APH-2 expression levels. β-Actin was used as a loading control. (C and E) Jurkat cells stably transduced with lentiviral vectors expressing HBZ (C) or APH-2 (E) were treated with 100 μg/ml cycloheximide for the indicated times. Cells were collected by centrifugation, washed in PBS, and lysed using NP-40 lysis buffer. Immunoblot analysis was performed to detect HBZ expression levels. β-Actin was used as a loading control. APH-2 was immunoprecipitated with a control normal rabbit IgG or APH-2 rabbit antiserum. The amount of immunoprecipitated APH-2 was then measured by immunoblot analysis.