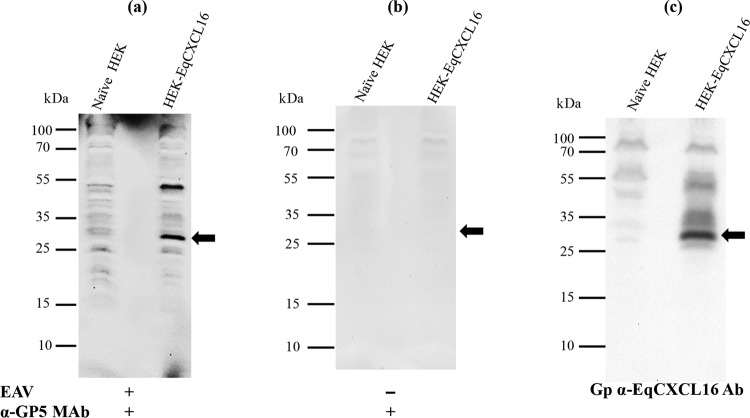
FIG 10.
Analysis of a direct in vitro interaction between purified EAV and EqCXCL16 protein by a combination of VOPBA and far-WB analysis. Stable HEK-EqCXCL16 or naive HEK-293T cell lysates were separated on a 12% SDS-polyacrylamide gel and transferred onto a PVDF membrane. The proteins in the membrane were denatured and renatured using sequentially decreasing concentrations of guanidine-HCl. The membranes were blocked and incubated either with purified EAV VBS at a concentration of 15 μg/ml in protein-binding buffer (a) or with protein-binding buffer only without purified EAV VBS (b). After they were washed, the membranes were incubated with anti-GP5 MAb 6D10 and developed using the ECL method. (a) Binding of EAV VBS to the EqCXCL16 protein (arrow). (c) Antibodies were stripped off the membrane shown in panel a and reprobed with Gp anti-EqCXCL16. As indicated by the arrow in panel c, EqCXCL16 was detected at the same position on the membrane where EAV GP5 was detected in panel a.