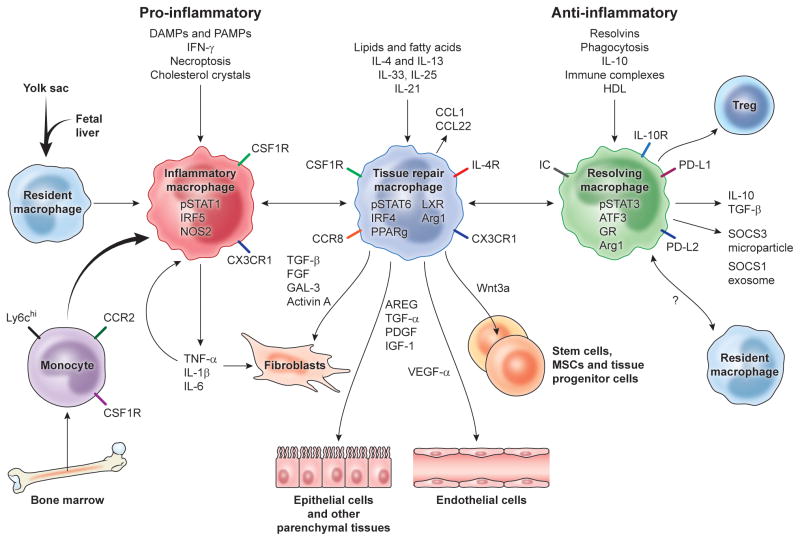
Fig. 1. Mechanisms driving major macrophage activation phenotypes in tissue repair, regeneration, and fibrosis.
In many tissues the resident tissue macrophage population is derived from the yolk sac and fetal liver during development but are complimented by inflammatory monocytes recruited from the bone marrow following injury. The recruited and resident macrophages undergo marked phenotypic and functional changes in response to DAMPs, PAMPs, growth factors, cytokines, and other mediators released in the local tissue microenvironment, with the dominant phenotypic variants depicted here regulating inflammation, tissue repair, regeneration, and resolution. Macrophages produce a variety of factors that stimulate the proliferation, differentiation, and activation of fibroblasts, epithelial cells, endothelial cells, and stem and progenitor cells that facilitate tissue repair. During the later stages of the repair process, they assume a regulatory pro-resolving phenotype that ensures the tissue damaging inflammatory response is suppressed and normal tissue architecture is restored. If the process is not controlled effectively, persistent inflammation and/or maladaptive repair processes can lead to tissue destructive fibrosis. In some cases, the recruited monocytes seed the tissues and adopt a resident macrophage phenotype, however the mechanisms that restore tissue homeostasis are still under debate. Damage associated molecular patterns (DAMPs), PAMPs (pathogen associated molecular patterns), Regulatory T cells (Treg), interferon-regulatory factor 5 (IRF5), nitric oxide synthase 2 (NOS2), Liver X receptor (LXR), Amphiregulin (AREG), Arginase-1 (Arg1), interferon regulatory factor 4 (IRF4), peroxisome proliferator-activated receptor gamma (PPARγ), fibroblast growth factor (FGF), galectin-3 (GAL-3), transforming growth factor (TGF), Immune complex (IC), glucocorticoid receptor (GR), transcription factor ATF3, silencers of cytokine signaling (SOCS).