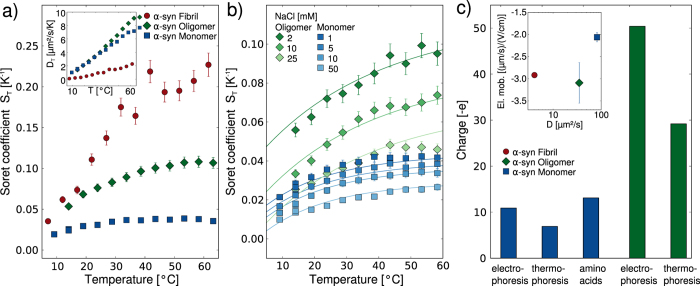
Figure 2. Thermophoretic characterization of three distinct α-synuclein species.
(a) The Soret coefficients, ST, of monomeric, oligomeric and fibrillar α-synuclein (in 1 mM Tris buffer at pH 7.4) as a function of temperature, showing their strong size-dependence. Inset: The thermal diffusion coefficient, DT = DST, as a function of temperature. (b) Fit of the temperature dependence of ST of α-synuclein monomers (blue) and oligomers (green) at different concentrations of added NaCl. The data are globally fitted to a model that includes the electrostatic effects relevant for thermophoresis and where the effective charges of the species and the Soret coefficient of the Tris ion are the only free parameters. (c) The charges determined from the fits in (b) compared with the charges determined from an analysis of the electrophoretic mobilities (supplementary section 6). For the monomer, the charge expected from the amino acid composition is also plotted. Inset: The free flow electrophoretic mobilities43 of fluorescently labeled monomeric, oligomeric and fibrillar α-synuclein (in 5 mM Tris buffer pH 7.4) are plotted against their diffusion coefficients (from FCS measurements42, and supplementary section 5).