Abstract
Background:
Malnutrition and tuberculosis (TB) tend to interact with each other. TB may lead to nutrition deficiencies that will conversely delay recovery by depressing immune functions. Nutrition support can promote recovery in the subject being treated for TB. The aim of this study was to evaluate the effectiveness of nutrition support on promoting the recovery of adult pulmonary TB patients with anti-TB drug therapy.
Methods:
English database of the Cochrane Controlled Trials Register, PubMed, EMBASE, and Chinese database of CBM, CNKI, VIP, and WANFANG were searched. Randomized controlled trials comparing nutrition support (given for more than 2 weeks) with no nutrition intervention, nutrition advice only, or placebo-control for TB patients being anti-TB treated were included. Two reviewers conducted data extraction, assessed the quality of the studies independently, and any discrepancies were solved by the third reviewer. Data were entered and analyzed by RevMan 5.2 software, and meta-analysis was done using risk ratios (RRs) for dichotomous variables and mean differences (MDs) for continuous variables with 95% confidence intervals (CIs).
Results:
A total of 19 studies (3681 participants) were included. In nutritional support for TB patients, pooled RR and its 95% CI of sputum smears- or culture-negative conversion rate and chest X-ray (CXR) absorption rate were 1.10 (1.04, 1.17) and 1.22 (1.08, 1.39), respectively, the pooled MD and its 95% CI of body mass index (BMI) and time of sputum smears or culture negativity were 0.59 (0.16, 1.2) and − 5.42 (−7.93, −2.92), respectively, compared with the control group. The differences in outcomes of CXR zone affected, TB score, serum albumin, and hemoglobin were not statistically significant (P = 0.76, 0.24, 0.28, and 0.20, respectively) between the intervention group and the control group. No systemic adverse events were recorded.
Conclusions:
During anti-TB course, nutrition support may be helpful in treatment of TB patients by improving both sputum smears- or culture-negative conversion rate and BMI, shortening the time of sputum conversion negative. Whether it can improve the final clinical effect, there still needs high-level quality studies to confirm in the future.
Keywords: Intervention, Nutrition Support, Tuberculosis
INTRODUCTION
Tuberculosis (TB) is a contagious bacterial disease caused by Mycobacterium tuberculosis, which most commonly affects the lungs. It is the leading killer from a single infectious agent worldwide, especially in Asia and Africa. In 2009,[1] in terms of a total number of TB cases in the world, India, China, Indonesia, and South Africa ranked first to four, respectively. The WHO 2014 reported[2] that TB remains a major public health problem in the world. An estimated 9 million people were infected with TB, including 1.1 million people who living with human immunodeficiency virus (HIV) at the same time. Each year, about 1.5 million people die of TB. More than 95% of the cases and deaths are in developing countries. Worldwide, the link between poor nutritional status and TB is well known in people with active TB, and weight loss is a well-recognized symptom of the disease.[3] In accordance with guideline 2013,[4] TB is associated with poverty, malnutrition, and poor immune function, and malnutrition can both precede and result from TB. For one thing, TB leads to malnutrition worse, protein-energy malnutrition, micronutrient deficiencies, be in a catabolic state, weight loss or low body mass index (BMI), loss of appetite, and fatigue.[5,6,7] For another perspective, malnutrition is believed to make people more susceptible to TB and to delay recovery by impairing cell-mediated immunity against M. tuberculosis, and thereby increasing the probability that latent TB will develop into active disease.[3,8] In addition, poor nutrition is known to influence the treatment of TB by reducing in some patients’ drug absorption of some key anti-TB drugs and by predisposing to drug-induced hepatotoxicity, which can lead to interruptions of therapy and may be fatal by itself.
In some trials, compared to the placebo group, consecutively receiving micronutrient is helpful to sputum smear, culture conversion negative rate, and other indicators or even make them significantly increased.[9,10,11,12] Also, specific nutritional diets (meals or food, such as enteral nutrition) provided for TB patients in hospital had received benefit results on both sputum smears conversion negative and gaining weight, including BMI. However, some studies[13,14] showed that the data from previous trials were inconsistent with the potential therapeutic effects on sputum smears or culture conversion negative when Vitamin A or zinc supplementation was given alone or combined with other micronutrients. In some developing countries,[15,16] the nutritional status for the majority of TB patients who combine with HIV infection or diabetes mellitus has shown a lower BMI than those who have no HIV or diabetes, and TB is even worse in severity and can accelerate the progression of HIV infection. The WHO suggests[17] that the primary predictors on body weight and sputum smear examination should be monitored for all TB patients during anti-TB treatment to evaluate their response to therapy. In addition, sputum smears or culture conversion negative is the major substituted marker of the efficacy of anti-TB chemotherapy.[18] However, no systematic review is available for nutrition support as adjunctive therapy to assess the effects on sputum smears or culture conversion negative time or rate and other nutritional indicators for TB patients during anti-TB course.
In this study, we aimed to solve above-mentioned conflicts and explore the evidence for the effectiveness of nutrition support in helping people to recover from TB and provide the reference basis for TB clinical treatment.
METHODS
Criteria for considering studies for this review (Participants, Interventions, Comparisons, and Outcomes)
The selected studies for analysis were all randomized controlled trials. We chose adult TB patients with sputum smear-positive for acid-fast bacilli or culture-positive for M. tuberculosis, with or without concurrent HIV infection, diabetes mellitus, and diagnosed malnutrition as types of participants; extra-pulmonary tuberculosis (PTB) patients and child patients (age <15 years old) were excluded. The types of interventions covered any nutritional support that given at least 2 weeks during the anti-TB treatment, including high-energy-protein dietary, enteral or intravenous nutrition support, dietary guidance of a special group, and/or single or multiple micronutrients. Actually, only the time of nutrition intervention and control measure more than 3 weeks was included in the data analysis. Placebo or no nutritional intervention was chosen as type of control. The primary outcome was intervention effect (time of sputum swears or culture-negative, rate of sputum swears- or culture-negative, or chest X-ray (CXR) improvement or absorption rate, or TB score) at 1, 2, 3, and 6 months. The secondary outcomes included change in BMI or weight gain 10% and changes in levels of markers of serum albumin (ALB) and/or hemoglobin. Only having at least one primary outcome studies with or without secondary outcomes were included in this review.
Search methods for identification of studies
English database of the Cochrane Controlled Trials Register, PubMed, EMBASE, and Chinese database of CBM, CNKI, VIP, WANFANG were searched from January 2003 to December 2013 using keywords of “nutritional support” or “nutrition therapy” or “nutrition intervention” or “micronutrient supplement” or “Protein-Energy malnutrition” or “nutrition assessment” and “tuberculosis and pulmonary” or “TB” or “PTB,” these keywords have already been verified by literature retrieval expert, the limitation of language was Chinese and English, full reports of potentially eligible trials were obtained, as well as the references of eligible studies were also searched. Two reviewers (Si ZL, Shen XB) independently selected all potentially eligible studies using a predesigned eligibility form based on the inclusion criteria. Any discrepancies or differences in opinion were solved by discussion, where necessary, we contacted with a third reviewer (Zhou YZ). We also documented the reasons for exclusion that did not satisfy the criteria.
Quality assessment in individual studies
Data extraction and quality assessment were performed independently by two reviewers (Si ZL, Kang LL). Five risk items of bias were used to assess the quality of included studies based on Cochrane Collaboration guidelines: Adequate sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data. When the information was missing or unclear, we tried to contact the authors. Any dissimilar comments were settled by discussion, in case of disagreement, one reviewer acted as arbitrator (Zhou YZ).
Statistical analysis
Statistical analysis was performed using Review Manager 5.2 software (Available at http://ims.cochrane.org/reman). For dichotomous data, we calculated each study risk ratios (RRs) for a binary variable. The mean difference (MD) was calculated for continuous data. All results were made with 95% confidence intervals (CIs). Results were stratified into subgroups according to different follow-up period with any nutrition support type and approach. We assessed potential heterogeneity of effect estimates within each group using I2 test, where I2 (%) >50% and P < 0.10 was considered significantly heterogeneous, then random effect model was applied to combine results from different trials, otherwise, fixed-effect model was used. If significant statistical heterogeneity was noted in the outcome, we conducted a sensitivity analysis to investigate the stability of the results by removing studies. Funnel plots for asymmetry were examined whether there was possible risk of bias. The test for overall effect of included studies with P values of <0.05 was considered statistically significant.
RESULTS
Description of studies
A total of 1055 articles were found using our search strategy. Of them, 19 conformed to the inclusion criteria, 7 were in Chinese, and 12 in English [Figure 1 and Table 1].[16,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] These 19 trials included 3681 participants in total. The studies were especially focused on adult patients; the age ranged from 15 to 70 years. Trials carried out in China included subjects with diabetes mellitus.[16,23,25] Studies in other countries included TB patients who were infected with the HIV.[20,21,30]
Figure 1.
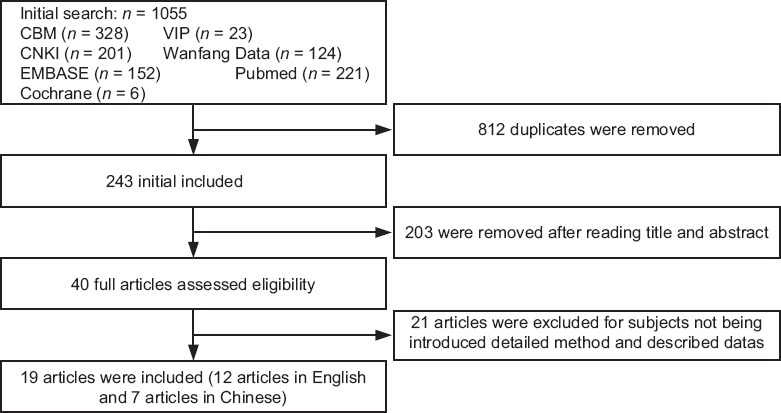
Flow diagram of study was completed with searching and selection process.
Table 1.
Characteristics of included studies
| Included study | Country | Sample size, n | Age, years | Interventions | Course | Outcome indictors | |||
|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | ||||
| Martineau 2011[19] | UK | 62 | 64 | Median = 30.7 | Median = 30.5 | Given VD3 2.5 mg + standard TB treatment | Placebo + standard TB treatment | 14, 28, and 42 days | Sputum culture conversion; hemoglobin, BMI |
| Ralph et al. 2013[20] | Indonesia | 99 | 101 | Median = 27 | Median = 28 | L-arginine 6.0 g + anti-TB therapy | Placebo + anti-TB therapy | 4, 8, 24 weeks | Sputum culture conversion, TB score; ≥10% weight gain |
| Cao[16] | China | 24 | 23 | 45–70 | 45–70 | Dietary intervention + 2EHRZ/4HR | Blank + 2EHRZ/4HR | 4 weeks | Sputum turning negative time |
| Wejse et al.[21] | Guinea- Bissau | 187 | 178 | 37 ± 13 | 38 ± 14 | 100,000 IU of VD3 + anti-TB treatment | Placebo + antiTB treatment | 2, 5, 8, 12 months | Sputum smears conversion, TB score; ALB, BMI |
| Villamor et al.[22] | Tanzania | 314 | 314 | 29.4 ± 9.0 | 30.6 ± 9.0 | Micronutrient supplementation + antiTB treatment | Placebo + antiTB treatment | 1, 8, 24 months | Culturenegative; body weight, ALB, hemoglobin |
| Guo and Xu[23] | China | 39 | 39 | 42.0 ± 9.8 | 46.0 ± 9.3 | Nutrition support therapy + conventional antiTB treatment | Blank + conventional antiTB treatment | 8 weeks | Sputum conversion rate; CT absorption rate |
| Ginawi et al.[24] | India | 29 | 32 | >15 | >15 | Micronutrient supplementation (Vitamine A 5000 IU + zinc 15 mg) + antiTB drugs on DOTs day | Placebo + antiTB drugs on DOTs day | 2, 6 months | Sputum smears negative; hemoglobin, ALB |
| Ma and Xie[25] | China | 80 | 80 | Mean = 39 | Mean = 39 | High-energy-protein dietary + Sanjiu enteral nutrition 30 g/d + conventional therapy | Blank + conventional anti-TB therapy | 3 weeks | The conversion rate of sputum culture; BMI, ALB |
| Visser et al.[26] | South Africa | 77 | 77 | Median = 30 | Median = 27 | Micronutrient (Vitamine A 200,000 IU and zinc 15 mg) + TB therapy | Placebo + anti-TB therapy | 2, 8 weeks | Sputum culture/smears conversion rate; ALB, hemoglobin |
| Salahuddin et al.[27] | Saudi Arabia | 132 | 127 | 27.8 ± 13.2 | 28.3 ± 14.1 | VD3 (60,000 IU) + DOTs | Placebo + DOTs regimen | 4, 8 and 12 weeks | Sputum smears conversion; resolution of chest radiograph; TB score; weight gain |
| Range et al.[28] | Tanzania | 251 | 248 | 35.5 ± 12.2 | 35.3 ± 12.3 | Multi-micronutrient supplement + TB therapy | Placebo + anti-TB therapy | 2, 4 and 8 weeks | Sputum smears/culture conversion; weight gain |
| Chandra[29] | India | 22 | 22 | 27–49 | 28–50 | Multivitamins supplementation + DOTs regimen | Placebo + DOTs regimen | 2, 3, 5 and 6 months | Sputum conversion, CXR zone; BMI |
| Schön et al.[30] | Ethiopia | 90 | 90 | 28.4 ± 1.1 | 26.5 ± 1.0 | Food supplement rich in arginine + treatment shortcourse (DOTs) clinic | Blank + shortcourse (DOTs) clinic treatment | 2, 8 months | Sputum smear conversion rate, CXR improvement; weight gain >10% |
| Tan et al.[31] | China | 26 | 26 | Median = 45 | Median = 42 | Energy essence mixture + 3DEC + X/6DE | Blank + 3DEC + X/6DE | 3, 6 and 9 months | Sputum changing negative rate; BMI; ALB |
| Pakasi et al.[32] | Indonesia | 66 | 86 | 30.1 ± 12.0 | 31.4 ± 10.4 | Micronutrient supplementation (Vitamine A 5000 IU + zinc 15 mg) + standard treatment | Placebo + standard antiTB treatment | 2, 6 months | Sputum conversion time; BMI, abnormalities on CXR |
| Wang and Xu[33] | China | 42 | 40 | 53.0 ± 3.8 | 55.0 ± 3.6 | High energyproteinvitamin diet nutrition therapy + TB therapy | Blank + anti-TB therapy | 3 weeks | The conversion rate of sputum culture; BMI, hemoglobin, ALB |
| Xu[34] | China | 200 | 198 | 42.6 ± 17.4 | 44.1 ± 15.9 | Dietary + highprotein enteral nutrition formulations + conventional therapy | Blank + conventional anti-TB therapy | 3 weeks | The conversion rate of sputum culture; BMI, ALB |
| Yang et al.[35] | China | 48 | 48 | Mean = 41.2 | Mean = 39.6 | Dietary treatment + conventional therapy | Blank + conventional anti-TB therapy | 2, 4 and 8 weeks | Sputum conversion rate; CT absorption rate |
| Yuan[36] | China | 60 | 40 | 16–50 | 16–50 | Dietary + DOTs | Blank + DOTs | 8 weeks | Sputum conversion rate |
E: Experimental group; C: Control group; ALB: Serum albumin; BMI: Body mass index; CT: Computed tomography, TB: Tuberculosis; CXR: Chest X-ray; DOTs: Directly-observed treatment strategy; VD3: Vitamin D3; 2EHRZ/4R: Ethambutol, isoniazid, rifampicin and pyrazinamide for 2 months, then rifampicin for 4 months; 3DEC: D means isoniazid + para-aminosalicylic acid, E means ethambutol, C means ciprofloxacin, 3 means 3 months; X/6DE: X means one of susceptibility drug, 6 means 6 months.
The included trials assessed a range of diverse nutritional supports. Interventions included a high-energy-protein dietary,[23,25,30,31,32,33,34,35,36] micronutrient supplements,[19,21,22,24,26,27,29,32] and arginine supplement.[19,30] Information on the doses and anti-TB regimens is shown in Table 1. The trials were carried out in China with blank as the control group, and the others were used for placebo as the control group. Supplement and placebo capsules were indistinguishable in appearance both externally and internally.
These 19 articles covered primary outcome that are the time of sputum smears or culture negativity, the conversion rate of sputum smears or culture; 7 of the articles reported the primary outcome of CXR improvement or absorption or TB score; and 15 of the articles contained some or part of nutritional indicators, such as BMI, weight gaining, hemoglobin, and ALB [Table 1].
Risk of bias in included studies
Eleven trials described an adequate method of generating a truly random allocation sequence, one trial depicted the method of generating a defective random allocation sequence,[34] and the other trials did not report that how group allocation sequences were generated although all were described as “randomized.”
Seven trials represented an adequate method of ensuring allocation concealment. The other trials did report insufficient information to determine if the allocation sequence was truly hidden from the person allocating participants to the treatment groups. Ten trials reported blinding of participants and personnel and 9 trials blinded participants in their group allocation using placebos and others with blank or dietary advice only.
We can directly come to conclusion that the qualities of included studies conducted in abroad were higher than those carried out in China. After contacting the author, 7 articles carried out in China got “unclear” in “random sequence generation” and no articles in China got “yes” in “allocation concealment,” while 11 studies carried out in abroad, only 3[24,29,32] got “no” in allocation concealment, and no trials in China got “yes” in “double blinding,” while conducted in non-China was 10 trials used for blinding.
The quality assessment of the included studies is shown in Table 2.
Table 2.
Quality assessment of included studies
| Included study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Martineau 2011[19] | L | L | L | L | H | L | U |
| Ralph 2013[20] | L | L | L | L | H | L | U |
| Cao[16] | U | H | H | H | L | L | U |
| Wejse et al.[21] | L | L | L | L | H | L | U |
| Villamor et al.[22] | L | L | L | L | H | L | U |
| Guo and Xu[23] | U | H | H | H | L | L | U |
| Ginawi et al.[24] | L | H | L | L | L | L | U |
| Ma and Xie[25] | L | H | H | H | L | L | U |
| Visser et al.[26] | L | U | U | U | H | L | U |
| Salahuddin et al.[27] | L | L | L | L | L | L | U |
| Range et al.[28] | L | L | L | L | H | L | U |
| Chandra[29] | U | H | L | L | L | L | U |
| Schön et al.[30] | L | L | L | L | H | L | U |
| Tan et al.[31] | U | H | H | H | L | L | U |
| Pakasi et al.[32] | L | H | L | L | H | L | U |
| Wang and Xu[33] | U | H | H | H | L | L | U |
| Xu[34] | H | H | H | H | L | L | U |
| Yang et al.[35] | U | H | H | H | L | L | U |
| Yuan[36] | U | H | H | H | L | L | U |
H: High risk; U: Unclear; L: Low risk.
Primary outcomes of the effects of interventions
Sputum smears- or culture-negative conversion rate of tuberculosis patients used any nutritional support method
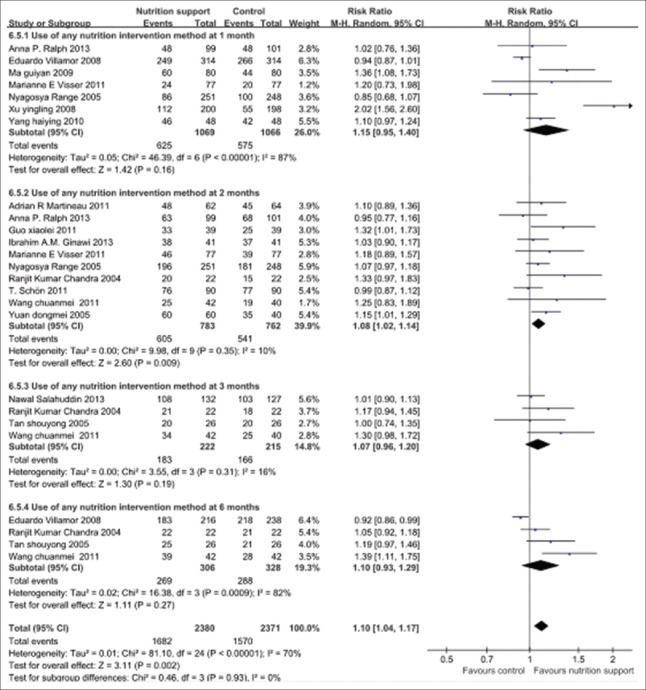
Seventeen trials reported on the primary outcome of sputum smears- or culture-negative conversion rate. According to the various points of follow-up, we selected four points (1, 2, 3, and 6 months, respectively) to reflect intensive phase and consolidation phase of the intervention effect for TB patients.
The pooled results of follow-up 1, 2, 3, and 6 months that given nutrition support group showed that the sputum smears- or culture-negative conversion rates were more remarkable increased than control group, in particular at 2 months, and the overall effects were significant different (P < 0.01). Furthermore, test for subgroup differences showed that there were not any differences among subgroups (P > 0.05). In this regard, the time and specific nutritional support approach impacted on the conversion rate of sputum smears or culture was limited. Significant statistical heterogeneity was noted in the outcome, therefore random effect model should be applied (P < 0.1 and I2 > 50%). The pooled RR and its 95% CIs were 1.10 (1.04, 1.17) [Figure 2].
Figure 2.
Sputum smears- or culture-negative conversion rates in tuberculosis patients with any nutrition support methods during follow-up. Vertical line indicates no difference between the two groups (Mantel-Haenszel vs. control). Squares indicate point estimates of risk ratio in each individual study, the size of the squares indicates the weight of the corresponding study in the meta-analysis, 95% confidence intervals of point estimates are demonstrated by horizontal lines. Pooled risk ratio and its 95% confidence interval are shown by a diamond shape.
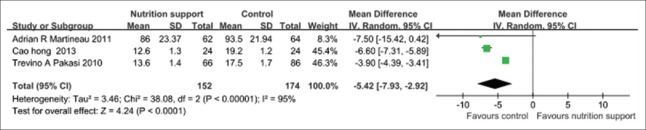
Time of sputum smears or culture negativity
In the pooled results of four trials [Figure 3], the time of sputum smears or culture negativity in nutrition support group was evidently shorter than that in the control group (P < 0.01). The random effect model should be applied (P < 0.1 and I2 > 50%), and the pooled MD and its 95% CI were −5.42 (−7.93, −2.92).
Figure 3.
Time of sputum smears or culture negativity in tuberculosis patients with any nutrition support methods.
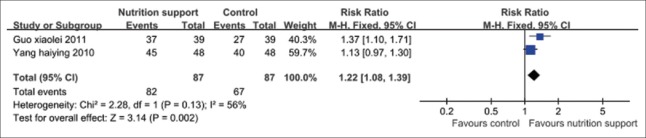
Chest X-ray absorption rates
Two trials[23,35] reported the effect of CXR absorption in high-energy and protein dietary was superior to dietary advice only or general diet group (P < 0.01). The fixed effect model should be applied, and the pooled RR and its 95% CI were 1.22 (1.08, 1.39) [Figure 4].
Figure 4.
Chest X-ray absorption rate in tuberculosis patients with high-energy and protein dietary.
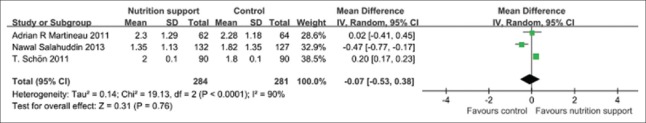
Chest X-ray zones affected
From the pooled results of three trials,[19,27,30] there was no any statistical difference (P > 0.05) in the CXR zones affected between micronutrient supplement group and the control group when micronutrient supplement was performed at follow-up 2 months [Figure 5].
Figure 5.
Chest X-ray zones affected in tuberculosis patients used micronutrient supplement.
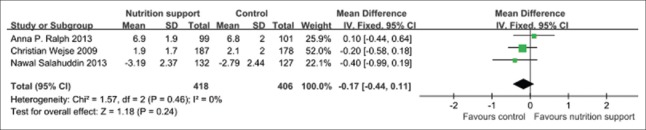
Tuberculosis score
In the pooled results of three trials,[20,21,27] there was no any statistical difference (P > 0.05) in TB score between micronutrient supplement group and the control group when micronutrient supplement was performed at follow-up 2 months. The fixed effect model should be applied, and the pooled MD and its 95% CI were −0.17 (−0.44, 0.11) [Figure 6].
Figure 6.
Tuberculosis score in tuberculosis patients used micronutrient supplement.
Secondary outcomes
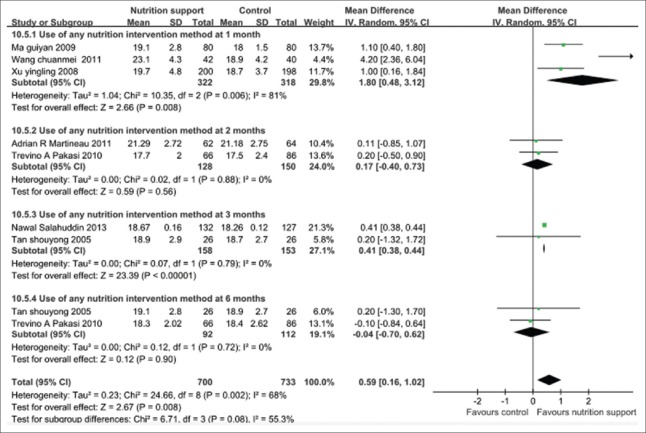
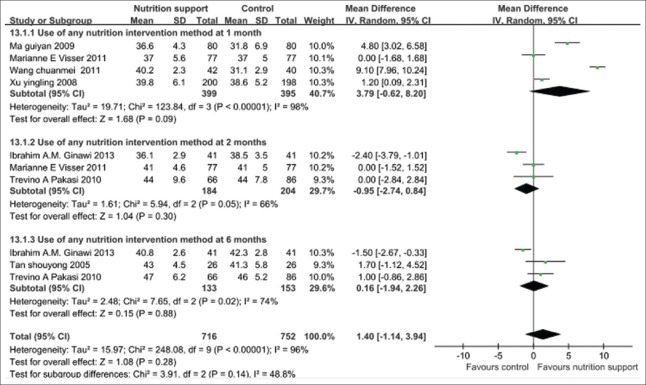
Body mass index changed during follow-up
The pooled results of following up 1, 2, 3, and 6 months showed that the nutrition support group gained significantly more BMI than the control group at different follow-up points, in particular, 1 month, and the overall effects with statistical significance (P < 0.01). However, test for subgroup differences showed that there was statistical significance among subgroups (P < 0.05). There was significant statistical heterogeneity in the outcome, the further analysis should be accounted for subgroup differences. The random-effects model should be used, and the pooled MD and its 95% CI were 0.59 (0.16, 1.02) [Figure 7].
Figure 7.
Body mass index gain in tuberculosis patients with any nutritional support method.
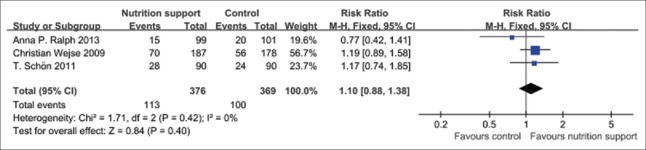
Weight gain 10% at 2 months
Three trials[20,21,30] reported that there was not any statistical difference (P > 0.05) in weight gain 10% between micronutrient supplement group and the control when micronutrient supplement was performed at follow-up 2 months. There was no significant statistical heterogeneity in the outcome; therefore, fixed effect model should be applied, and the pooled RR and its 95% CI were 1.10 (0.88, 1.38) [Figure 8].
Figure 8.
Weight gain 10% in tuberculosis patients with micronutrient support method.
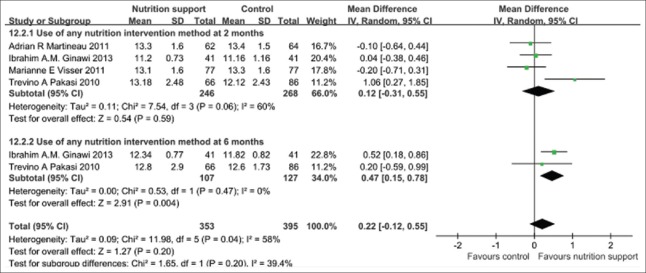
Hemoglobin changed during follow-up
From the pooled results of following up 2 months, 6 months after giving micronutrient supplement compared with the control groups showed that there was no any statistical difference (P > 0.05) in improving hemoglobin between micronutrient supplement group and the control group when micronutrient supplement was performed at follow-up 2 and 6 months. However, test for subgroup differences showed that there was no any statistical significance among subgroups (P > 0.05). There was no any significant statistical heterogeneity in the outcome, and then fixed effect model should be applied. The pooled MD and its 95% CI were 0.22 (−0.12, 0.55) [Figure 9].
Figure 9.
Hemoglobin increase in tuberculosis patients with micronutrient support method.
Meta-analysis of serum albumin increase in tuberculosis patients during follow-up
The pooled results of following up 1 month, 2 months, 6 months with any nutrition support method compared with the control groups showed that there was no any statistical difference (P > 0.05) in increasing ALB between nutrition support group and the control during follow-up points at 2 and 6 months. However, test for subgroup differences showed that there were no statistically significant differences among subgroups (P > 0.05). There was no any significant statistical heterogeneity in the outcome, and then fixed effect model should be used. The pooled MD and its 95% CI were 1.40 (−1.14, 3.94) [Figure 10].
Figure 10.
Meta-analysis of serum albumin increase in tuberculosis patients with any nutrition support method during follow-up.
Sensitivity analysis and funnel plots analysis
Sensitivity analysis
In the studies of sputum smears- or culture-negative conversion rate, after removing two studies,[22,34] the result of RR was 1.08 (95% CI: 1.03–1.14). The two studies either included or excluded, the result of P value is the same. To further analyze the sensitivity of included studies, two studies were excluded because of dissimilar results or high risk on generating a truly random allocation sequence. For time of sputum smears or culture negativity and BMI, the summary of MD was −5.42 (−7.88, −2.59) and 0.54 (0.07, 1.01) after removing either of the two studies,[19,34] respectively, which were still near to the results before they were excluded. Regarding the CXR absorption rate, we could not analyze the sensitivity because of there are only two studies. Therefore, regardless of the nutrition support type and approach, the results of the sensitivity analysis demonstrated that the results of our study were steady and believable [Table 3].
Table 3.
Sensitivity analysis of comparing with the random effect results after removing the dissimilar trials
| Study indicator | Excluded study | Before excluded | P | After excluded | P |
|---|---|---|---|---|---|
| Random effect RR/MD (95% CI) | Random effect RR/MD (95% CI) | ||||
| Sputum smears- or culture-negative rate | Villamor et al.[22] Xu[34] | 1.10 (1.04, 1.17) | 0.002 | 1.08 (1.03, 1.14) | 0.002 |
| Time of sputum smears or culture negativity | Martineau 2011[19] | –5.42 (−7.93, −2.92) | <0.001 | –5.42 (−7.88, −2.59) | 0.0001 |
| Change in BMI | Xu[34] | 0.59 (0.16, 1.02) | 0.008 | 0.54 (0.07, 1.01) | 0.030 |
CI: Confidence interval; RR: Risk ratios; MD: Mean differences; BMI: Body mass index.
Funnel plots analysis of sputum smears- or culture-negative conversion rate
Figure 11 shows that the funnel plots were focused on top, and there was no obvious symmetrical, especially at 1 month, which may mainly cause by publication bias.
Figure 11.
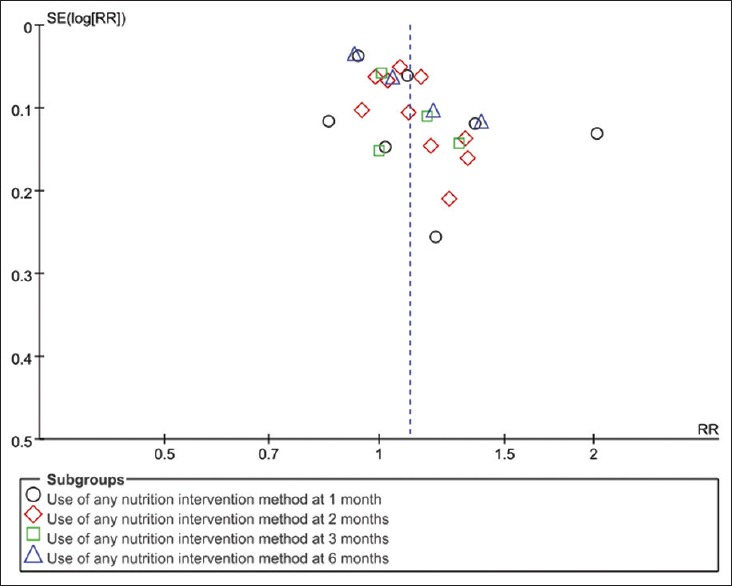
Funnel plots analysis of sputum smears- or culture-negative conversion rate.
DISCUSSION
In this review, we attempted to evaluate the evidence for the effectiveness of nutrition support in assisting PTB. The 19 trials, in which a total number of 3681 TB patients involved, were conducted in a variety of settings including different layer income countries crossing Africa, South-East Asia, and Western Pacific, which mainly concentrated in Africa and Asia. Test for all subgroup differences showed that there were not any statistical differences among subgroups when provided any nutrition support type and approach for TB patients. Therefore, the effect of any nutrition support type and approach was similar for TB patients when compared with the control group. So, the study only considered two main factors (intervention time and effect size) without any complicating co-factors between diverse groups.
The current meta-analyses found that the impact of improved sputum-negative conversion rates and BMI, shortened the time of sputum-negative conversion, is well-recognized. All above results were statistically significant compared with control groups during long-term anti-TB. However, significant statistical heterogeneity was noted in both outcomes. Also, CXR absorption in high-energy and protein dietary was superior to dietary advice only or general diet group with statistically significant. The main factors of these findings might be considered. It is well known that the TB infectious source are mainly sputum smear- or culture-positive TB patients, especially with smear-positive PTB possess stronger infectious. Sputum conversion negative was the main result of anti-TB treatment. This review showed that nutritional support may accelerate the process since the time of sputum conversion negative for nutritional support was shorter than control as shown in Figure 3. The possible reasons for this result as follow, immune function of the body might be weak when M. tuberculosis invades, and commonly losing of appetite during anti-TB course, inadequate intake of food and micronutrients might lead to malnutrition, especially in the first 2 months-intensive phase. So, continuously providing nutrition support (protein and energy intake and intake of micronutrients) not only enhances the immune response function but also makes the cell-mediated immunity fight the M. tuberculosis, which can be maintained or increased in nutritional status.
It is well known that low BMI (<18.5 kg/m2) is an established indicator for energy deficiency and malnutrition also influences the capacity of the cell-mediated immunity, which is the principle host defense against MTB.[37,38,39] Receiving an adequate high-energy-protein diet added enteral or intravenous nutrition group during anti-TB treatment in hospital may directly result in BMI gaining more significantly than the control at 1 and 3 months. It is possible that an adequate diet, containing all essential macro-and micro-nutrients, is beneficial for TB patients to improve the BMI, and it might be the reason why test for subgroups differences showed no significant difference. Similar meta-analysis studies show[40,41] that short- and long-term use of specific nutritional support (oral supplements and tube feeds) for patients with diabetes are associated with improved glycemic control compared with standard formulas, and this may have implications for reducing chronic complications of diabetes such as cardiovascular events.
Macro-nutrition and enteral or intravenous nutrition showed statistical significance on CXR absorption rate. However, only two studies account for it, and the quality of the two trials is low. Is it the true effect or just a distorted estimate because of poor sample size needs to be considered? Similar results in individual studies with low quality distributed relatively in some areas. It is worth further research and more studies of high quality are warranted.
The meta-analysis of TB score, hemoglobin, and ALB with micronutrient supplement does not show any statistical significance during anti-TB treatment period. A couple of factors may account for these: First, TB score,[42] based on symptoms and relate to signs, is a newly developed tool aimed at assessment of change in clinical state in patients. A high TB score correlates well with mortality, and low TB scores correlate with favorable outcomes, cure, and completed treatment rate. Micronutrient intake, especially intake of vitamins and minerals, has been associated with immune response, so it may have a weak relationship to improve the symptoms. Perhaps, it may relate to the insufficient number of included studies and a short intervention period. Second, a large majority of included patients are under-nutrition and being in catabolic state, especially during anti-TB drug treatment, which may be an issue that related to the extremely high dose requirement compared with healthy people, especially combined HIV and diabetes. For another, in accordance with previous studies,[43,44] the concentrations of ALB have the potential to be a useful diagnostic and prognostic marker for TB, however, malnutrition, chronic inflammation, or hepatic disease may lead to reduced ALB, and then relying on nutritional support to improve ALB of PTB patients is not obvious. Hemoglobin synthesis is an impediment when the M. tuberculosis invades, because the metabolic cycle of hemoglobin is taking longer than normal. So, the utility of nutrition support to improve the ALB and hemoglobin in common clinical practice is not straightforward.
Nutrition support is safe and well tolerated without any systemic adverse events reported in the included studies. The quality assessment showed this notion in high, especially the non-China trials. Furthermore, the results of sensitivity analysis showed the same result between after removing trials and before, thereby the results are reliable. There are still several factors that may lead to heterogeneity such as publication bias, carrying out in different settings with limited technological examination. The combination of nutrient support and anti-TB treatment show that it is beneficial on sputum conversion negative rates, time and BMI.
There are still several limitations in our review: First, with restriction on language, only Chinese and English studies are included, may lead to language bias. Second, there are still some references missing, poor sample size, gray literature, which may lead to publication bias. Third, the follow-up time points varied across studies and were not in complete accordance with each other. So, we choose the follow-up period that can represent the intensive phase and consolidation period to divide further time points, perhaps it is not applied to some trials. Moreover, the high-energy-protein food or intravenous nutrition and the multi-dose use of micronutrients in different countries are not standardized, so the optimum dosing schedule has still to be determined. In addition, it is worth noting that there is still no evidence on cure of TB treatment outcomes when using nutrition support as an addition to standard care. It is just beneficial to improve the sputum-negative conversion, shorten the conversion time, and increase the BMI.
In conclusion, there is sufficient evidence to assess the effect on sputum conversion negative rate, time, and BMI. During anti-TB course, regardless of its type and approach, nutrition support may be useful for the treatment of TB patients by improving both sputum conversion negative rate and BMI, shortening the time of sputum conversion negative. Based on the present evidence, there still needs higher quality trials to test about the effect of nutrition support on CXR absorption rate. Moreover, in terms of improving the TB score, further evidence that accounts for ALB and hemoglobin should be considered as well.
It is necessary for further studies to concentrate on any nutrition types for which encouraging results have already been demonstrated, including high-energy-protein food, multiple micronutrients or in combination with single micronutrient (zinc, Vitamin A, Vitamin D, and so on), as well as other meal replacements, conducted in areas where TB is prevalent to evaluate the effect on CXR absorption rate or zone changes, TB score, ALB, and hemoglobin. These trials should have sufficient sample sizes and at least moderate quality to detect what may be relatively final clinical effect. Moreover, it is needed for further trials to include participants who are HIV-positive or have complication diseases.
Financial support and sponsorship
This work was supported by grants from the Social Research Project in Guizhou Province (No. SY (2010) 3056 and (2012) 3112) and the Second 2011 Collaborative Innovation Center for Tuberculosis Prevention and Cure in Guizhou Province (Incubation Project).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely appreciate the help from teachers Jia Ma, Xiao-Li Shen and Lu Wang for providing us with literature search to the text.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Geneva: World Health Organization; 2009. World Health Organization. Global Tuberculosis Control 2009 (M) [Google Scholar]
- 2.Geneva: World Health Organization; 2014. World Health Organization. Global Tuberculosis Control. Report 2014 (M) [Google Scholar]
- 3.van Lettow M, Fawzi WW, Semba RD. Triple trouble: The role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 4.Geneva: World Health Organization; 2013. World Health Organization. Guideline: Nutritional Care and Support for Patients with Tuberculosis. [PubMed] [Google Scholar]
- 5.Dodor E. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. 2008;42:22–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: Evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–98. [PubMed] [Google Scholar]
- 7.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96:291–4. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 8.Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34:153–7. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 9.Armijos RX, Weigel MM, Chacon R, Flores L, Campos A. Adjunctive micronutrient supplementation for pulmonary tuberculosis. Salud Publica Mex. 2010;52:185–9. doi: 10.1590/s0036-36342010000300001. [DOI] [PubMed] [Google Scholar]
- 10.Villamor E, Fawzi WW. Effects of Vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb AL, Villamor E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr Rev. 2007;65:181–217. doi: 10.1111/j.1753-4887.2007.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 12.Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr. 2005;59:960–8. doi: 10.1038/sj.ejcn.1602201. [DOI] [PubMed] [Google Scholar]
- 13.Lawson L, Thacher TD, Yassin MA, Onuoha NA, Usman A, Emenyonu NE, et al. Randomized controlled trial of zinc and Vitamin A as co-adjuvants for the treatment of pulmonary tuberculosis. Trop Med Int Health. 2010;15:1481–90. doi: 10.1111/j.1365-3156.2010.02638.x. [DOI] [PubMed] [Google Scholar]
- 14.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Daily multi-micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV-uninfected but not HIV-infected patients in Mwanza, Tanzania. J Nutr. 2011;141:685–91. doi: 10.3945/jn.110.131672. [DOI] [PubMed] [Google Scholar]
- 15.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. The effect of energy-protein supplementation on weight, body composition and handgrip strength among pulmonary tuberculosis HIV-co-infected patients: Randomised controlled trial in Mwanza, Tanzania. Br J Nutr. 2012;107:263–71. doi: 10.1017/S0007114511002832. [DOI] [PubMed] [Google Scholar]
- 16.Cao H. Diet and nutrition support of patients with pulmonary tuberculosis concomitant with diabetes mellitus (in Chinese) Chin Med Pharm. 2013;3:211–12. [Google Scholar]
- 17.4th ed. Geneva: World Health Organization; 2010. World Health Organization. Treatment of Tuberculosis: Guidelines. [PubMed] [Google Scholar]
- 18.Liu Z, Shilkret KL, Ellis HM. Predictors of sputum culture conversion among patients with tuberculosis in the era of tuberculosis resurgence. Arch Intern Med. 1999;159:1110–6. doi: 10.1001/archinte.159.10.1110. [DOI] [PubMed] [Google Scholar]
- 19.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose Vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet. 2011;377:242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E, et al. L-arginine and Vitamin D adjunctive therapies in pulmonary tuberculosis: A randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8:e70032. doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 22.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197:1499–505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo XL, Xu Y. Treatment of pulmonary tuberculosis with diabetes efficacy of nutritional support (in Chinese) J Clin Pulm Med. 2011;16:1732–3. [Google Scholar]
- 24.Ginawi IA, Ahmed MQ, Ahmad I, Al-Hazimi AM. Effect of zinc and Vitamin A supplement along with inter-tubercular treatment in pulmonary tuberculosis in North India patients. Int J Pharm Sci Res. 2013;4:3426–43. [Google Scholar]
- 25.Ma GY, Xie JX. Survey and analysis on nutritional status of patients with pulmonary tuberculosis and efficacy of nutritional support (in Chinese) J Med Forum. 2009;30:61–2. [Google Scholar]
- 26.Visser ME, Grewal HM, Swart EC, Dhansay MA, Walzl G, Swanevelder S, et al. The effect of Vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: A randomized controlled trial. Am J Clin Nutr. 2011;93:93–100. doi: 10.3945/ajcn.110.001784. [DOI] [PubMed] [Google Scholar]
- 27.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of Vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Range N, Andersen ÅB, Magnussen P, Mugomela A, Friis H. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: A randomized controlled trial in Mwanza, Tanzania. Trop Med Int Health. 2005;10:826–32. doi: 10.1111/j.1365-3156.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 29.Chandra RK. Nutrient supplementation as adjunct therapy in pulmonary tuberculosis. Int J Vitam Nutr Res. 2004;74:144–6. doi: 10.1024/0300-9831.74.2.144. [DOI] [PubMed] [Google Scholar]
- 30.Schön T, Idh J, Westman A, Elias D, Abate E, Diro E, et al. Effects of a food supplement rich in arginine in patients with smear positive pulmonary tuberculosis – A randomised trial. Tuberculosis (Edinb) 2011;91:370–7. doi: 10.1016/j.tube.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Tan SH, Chen HG, Guan YH, Chen ZC. The use of nutrition-assisting system in treating replasing pulmonary tuberculosis (in Chinese) J Clin Pulm Med. 2005;10:147–50. [Google Scholar]
- 32.Pakasi TA, Karyadi E, Suratih NM, Salean M, Darmawidjaja N, Bor H, et al. Zinc and Vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr J. 2010;9:41. doi: 10.1186/1475-2891-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CM, Xu LD. An evaluation of effects of nutrition therapy on pulmonary tuberculosis (in Chinese) J Clin Pulm Med. 2011;16:215–6. [Google Scholar]
- 34.Xu YL. Investigation on the nutritional statesand nutritionaltherapy in 398 patients with pulmonary tuberculosis (in Chinese) J Chin Antituberc. 2008;30:335–7. [Google Scholar]
- 35.Yang HY, Liang JQ, Xiong XL, Peng Y. The efficacy of dietary guidance on drug-resistant with smear-positive pulmonary tuberculosis patients (in Chinese) J Qilu Nurs. 2010;16:70–1. [Google Scholar]
- 36.Yuan DM. Effects of dietary nutrition on tuberculosis patients with chemotherapy (in Chinese) Today Nurse. 2005;7:30–1. [Google Scholar]
- 37.Sultan KM, Alobaidy MW, AL-Jubouri AM, Naser AA, AL-Sabah HA. Assessment of body mass index and nutritional status in pulmonary tuberculosis patients. J Fac Med Baghdad. 2012;54:204–8. [Google Scholar]
- 38.Zheng Y, Ma A, Wang Q, Han X, Cai J, Schouten EG, et al. Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus. PLoS One. 2013;8:e80122. doi: 10.1371/journal.pone.0080122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez-Uria G, Midde M, Pakam R, Naik PK. Diagnostic and prognostic value of serum albumin for tuberculosis in HIV infected patients eligible for antiretroviral therapy: Datafrom an HIV cohort study in India. Bioimpacts. 2013;3:123–8. doi: 10.5681/bi.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care. 2005;28:2267–79. doi: 10.2337/diacare.28.9.2267. [DOI] [PubMed] [Google Scholar]
- 41.Coursin DB, Connery LE, Ketzler JT. Perioperative diabetic and hyperglycemic management issues. Crit Care Med. 2004;32(4 Suppl):S116–25. doi: 10.1097/01.ccm.0000115623.52021.c0. [DOI] [PubMed] [Google Scholar]
- 42.Wejse C, Gustafson P, Nielsen J, Gomes VF, Aaby P, Andersen PL, et al. A clinical score system for monitoring tuberculosis in a low-resource setting. Scand J Infect Dis. 2008;40:111–20. doi: 10.1080/00365540701558698. [DOI] [PubMed] [Google Scholar]
- 43.Al-Muhammadi MO, Al-Shammery HG. Studying some hematological changes in patients with pulmonary tuberculosis in Babylon governorate. Med J Babylon. 2011;8:608–17. [Google Scholar]
- 44.Tabarsi P, Chitsaz E, Moradi A, Baghaei P, Farnia P, Marjani M, et al. Treatment outcome, mortality and their predictors among HIV-associated tuberculosis patients. Int J STD AIDS. 2012;23:e1–4. doi: 10.1258/ijsa.2009.009093. [DOI] [PMC free article] [PubMed] [Google Scholar]