Abstract
Laser-based microdissection facilitates the isolation of specific cell populations from clinical or animal model tissue specimens for molecular analysis. Expression microdissection (xMD) is a second-generation technology that offers considerable advantages in dissection capabilities; however, until recently the method has not been accessible to investigators. This protocol describes the adaptation of xMD to commonly used laser microdissection instruments and to a commercially available handheld laser device in order to make the technique widely available to the biomedical research community. The method improves dissection speed for many applications by using a targeting probe for cell procurement in place of an operator-based, cell-by-cell selection process. Moreover, xMD can provide improved dissection precision because of the unique characteristics of film activation. The time to complete the protocol is highly dependent on the target cell population and the number of cells needed for subsequent molecular analysis.
INTRODUCTION
Development of the protocol
Since its original invention in the 1990s, laser microdissection technology has evolved substantially, with the development of several commercially available dissection instruments and the incorporation of computerized platforms and image analysis software1–4. In parallel, protocols for analyzing small amounts of DNA, RNA and protein have improved, facilitating quantitative and in-depth analysis of cellular genomes, transcriptomes and proteomes5–10. Current laser-based instruments have successfully advanced the molecular pathology field, and microdissection studies are now routinely used by a wide spectrum of laboratory- and clinic-based researchers. However, this field has certain challenges, such as the need for improved precision and the requirement for relatively large amounts of biomolecules for some downstream analysis platforms.
Expression microdissection (xMD) represents a conceptual and practical advance that offers substantive improvements for laser dissection based on operator-independent selection of cells using molecular targeting11. For xMD, a clear ethylene vinyl acetate (EVA) film is applied to a histological slide containing immunohistochemically (IHC) stained cells and exposed to a light source that irradiates the entire tissue section (Fig. 1a). The light energy is absorbed by the chromogen, causing focal heat activation of the dissection film and bonding it to target cells. Removal of the film from the histological section procures immunostained cells for subsequent molecular analysis.
Figure 1.
Schematic diagrams of xMD and LCM. (a) Schematic showing the principle of xMD. Target cells are selectively immunolabeled, covered with an EVA film and the entire tissue section is irradiated. The transient temperature increases focally at the site at which the chromogen melts the EVA polymer and bonds it to underlying target cells. No microscopic visualization or operator-based cell selection is required during the xMD process. (b) Schematic showing the principle of standard laser capture microdissection. First, under direct microscopic visualization, the operator identifies target cells in the tissue section. Then, the laser is manually fired at the targets in a one-by-one manner to generate a heat transient that melts EVA and bonds it to the underlying cells.
Our original proof-of-concept xMD publication used a PixCell II laser microscope for dissections, as well as a prototype instrument with a motorized stage and a fixed 400-mW laser source, using clear EVA films that were modified in-house at the NIH11. Tissues processed with various fixatives were analyzed in the initial study, including formalin-fixed, paraffin-embedded (FFPE) samples; ethanol-fixed, paraffin-embedded (EFPE) specimens; and cryopreserved tissue. Targeting probes included antibodies against proliferating cell nuclear antigen, cytomegalovirus, cytokeratin AE1/AE3, CD3, prostate-specific antigen, desmin, S-100, glyceraldehyde 3-phosphate dehydrogenase, E-cadherin and smooth muscle actin. In a second xMD study, we used a PixCell II system and in-house xMD films to dissect smooth muscle actin-positive and cytokeratin AE1/AE3-positive cells for subsequent epigenetic analysis, and then a third study used a similar xMD strategy and targeting antibodies against CD31 and Factor VIII to analyze endothelial cells in normal and cancerous tissue12,13. The protocol presented here is based on these previous studies, but it has been adapted to several different laser sources to make xMD technology accessible to the biomedical research community.
Comparison with other methods
As opposed to xMD, standard laser capture microdissection (LCM) is based on visual identification of target cells through a microscope1,2. A histological section is stained, and then a dye-containing EVA thermoplastic polymer is applied onto the slide (Fig. 1b). An IR laser is activated under microscopic visualization and aimed at cells under the operator’s guidance. The light energy is absorbed by the dye in the polymer causing it to partially melt and bond to the targets1,2,14. Subsequent removal of the dissection film from the tissue procures the bonded cells and they are placed into an appropriate buffer for molecular analysis. For both xMD and standard LCM, the IR energy is mostly absorbed by the chromogen or dye and not by the tissue; thus, there is little or no damage to the cells and the quality of the recovered biomolecules is related to the effects of upstream tissue processing and not to the dissection process itself 1,2,14–16.
Immuno-based laser dissection is a follow-on technique developed by Fend et al.17 that uses immunotargeting to improve visualization of cells of interest. In this approach, IHC or immunofluorescence is used as a visualization tool to identify specific cells that the laser operator then procures under microscopic visualization in a one-by-one manner. More recently, the incorporation of image analysis programs into laser microscopes has facilitated dissection of immunolabeled target cells via automatic recognition software; however, visual identification of positive cells by a human operator is typically needed to ensure proper cell selection. Immuno-based laser dissection has been used for numerous published articles, including epigenetic analysis, tumor micro-environment studies and neuroscience research17–25. Moreover, improved IHC protocols allow for the use of specialized types of films such as polyethylene naphthalate membrane slides22.
The adaptation of xMD to available instruments provides a new dissection tool for investigators studying phenotype or molecularly defined cells in histological sections. Our expectation is that xMD will extend microdissection capability into new areas of biological inquiry and that the technique will be complementary to, rather than competitive with, existing approaches. There are two important advantages of xMD that facilitate dissection-based analyses: precision and throughput.
The precision of microdissection using xMD is improved because of the unique physical properties of film-cell interaction. As activation of EVA is strictly a consequence of the focal temperature increase generated by the adjacent chromogen on target cells (as opposed to the size of the targeting laser beam in standard laser dissection), the area of activation and subsequent capture are reduced, thus allowing a higher resolution microdissection such as endothelial cells or nuclei11,13. Importantly, because xMD is based on a probe for dissection, all targeted cells or targeted organelles are rapidly procured in an operator-independent manner. In contrast, performing precise dissections using standard laser microscopes is an extremely challenging and tedious process that, even when successful, recovers only a few targets and scant biomolecules with limited use in molecular assays. Because xMD can procure all of the probe-targeted cells or organelles in a histological section, whether it contains 10 targets or 106 targets, relatively large amounts of DNA, RNA or protein can be generated from precisely dissected cells or organelles.
With respect to throughput, standard laser dissection and immuno-LCM work well for downstream molecular assays that either require small amounts of input material or are nucleic-acid based and amenable to amplification methods. For many assays, though, a significant increase in the number of dissected cells is advantageous; proteomic analysis is one such example4. Moreover, even for amplification-based protocols the general rule of thumb in the dissection field is ‘the more cells the better’, as the molecular data are typically improved as the amount of starting material increases, and the depth of the analysis is increased as more moderate- and low-abundant molecules can be measured. Thus, even some amplification-based studies (except for mRNA from cryosections, see below) may be improved by taking advantage of the high-throughput capability of xMD, especially when performed in concert with an automated immuno-staining instrument that rapidly processes slides.
However, an important caveat for xMD technology is the effect that IHC staining has on the biomolecules in tissue sections24,25. The immunostaining process includes several incubation and wash steps, followed by enzymatic-based colorimetric labeling. Currently, xMD can be successfully applied to DNA analysis, proteomics and to the study of mRNA and microRNA in archival samples; however, measurement of mRNA in frozen tissue sections is compromised by the immunostaining procedure and is challenging to perform using any of the immuno-based dissection techniques (see Box 1)24,25. Although this is a limitation of the current xMD method, this difficulty is mitigated for investigators by the availability of transcriptome amplification methods that can be used to analyze the small number of cells procured in a standard laser dissection study. The unique niche for xMD is likely to be microRNA and mRNA measurements from archival FFPE samples in which a substantive increase in starting material, of an order of magnitude or more, is often required (beyond that used for frozen sections) because of the fragmentation of nucleic acids that occurs during the upstream tissue processing steps.
BOX 1. EXTRACTION FROM xMD FILMS.
Standard buffers and kits used for laser-microdissected samples can be used to extract captured tissue from xMD films. The exact buffer or kit depends on the biomolecule(s) of interest and the method of tissue preservation.
For DNA, we use the QIAamp DNA Micro kit (Qiagen).
For RNA extraction, we use the PicoPure kit (Life Technologies).
For denatured protein analysis (1D-PAGE), we use the Novex Tris-glycine SDS sample buffer (2×; Life Technologies) with gentle heating (55 °C) and agitation. Do not heat the film in the SDS buffer above 80 °C because the film will melt and compromise sample recovery.
For mild protein analysis (2D-PAGE), we use the Total Protein Isolation Kit (ITSI Biosciences); however, note that the recovery of proteins from immunostained tissue sections varies depending on the tissue type and staining method. In general, protein profiling after xMD is better accomplished using mass spectrometry.
For protein mass spectrometry, we use a protocol described by Johann et al.9.
Following xMD, the ethylene vinyl acetate film with transferred cells should be placed into a 1.5-ml Eppendorf tube containing sufficient extraction buffer to cover the film while incubating the tube with regular agitation using an Eppendorf themomixer or an orbital heater.
Ultimately, investigators need to select the proper dissection tool for their particular study on the basis of the goals of the analysis, the abundance level of the molecule(s) of interest, the number of target cells in the tissue available for dissection and the quality of specimens related to tissue processing. In the LCM Core Laboratory at NIH, we routinely recommend that investigators perform a set of pilot tests on their samples before commencing a large study to assess each variable and select the proper dissection strategy.
Experimental design
In this paper, we describe protocols for using xMD technology on three commercially available laser dissection instruments and on a handheld laser device. The specific goal was to adapt xMD to them with minimal modifications. Among the commercial dissection instruments we tested, the ArcturusXT and Veritas systems are preferred because their motorized stages and auto-selection software facilitate the rapid irradiation of whole slides.
Slide preparation and immunohistochemistry
Similar to standard laser dissection, xMD can be used to recover cells from histological sections of frozen tissue or from paraffin-embedded tissue fixed with formalin or ethanol. The microtome- or cryosections should be mounted on a charged slide (see PROCEDURE for more details) and IHC stained with chromogen 3,3′-diaminobenzidine (DAB)11. The slide should not be counterstained afterward and no cover slip should be placed on the tissue section before xMD. The general rule of thumb regarding IHC intensity is that strongly stained cells will be efficiently dissected, but weakly stained cells or any cells or organelles with a ‘blush background’ will generally not be procured. There is a distinct threshold of film activation that requires strong staining; therefore, the primary antibody concentration used for IHC should be titrated accordingly.
Preparation of the EVA polymer
To improve cell dissection efficiency, we tested variations in film-tissue pressure and film preheating, as good contact between the EVA polymer and the tissue section is essential for xMD. For the original prototype instrument at NIH, we accomplished this by using an air-bearing jet near the laser diode output that served to mechanically enhance contact of the film overlaying the tissue with the cells to be dissected. However, as we wanted to adapt xMD technology to standard laser instruments and devices without making substantive changes in them, we tested two strategies to improve the EVA/tissue contact: first, application of pressure, and second, application of heat. The increased malleability of EVA from heating the polymer films, combined with the use of gentle pressure applied using a roller, proved to be the optimal means to ensure close contact with the tissue (see PROCEDURE for additional details). The best heating conditions for pretreatment of the EVA polymer include a temperature ranging from 60 °C to 80 °C for 2–5 min, although the optimal temperature and time vary depending on the composition of the EVA film (see PROCEDURE and Box 2). As shown in Figure 2, this approach allowed selective capture of breast cancer cells immunolabeled with antibodies against estrogen receptors (Fig. 2a–e) and dissection of prostate epithelium using targeting of cytokeratin AE1/AE3 (Fig. 2f,g). With a relatively low laser power, target cells were precisely bonded to the film, resulting in a high-resolution dissection.
BOX 2. EVA FILM FOR xMD.
EVA is a copolymer of ethylene and vinyl acetate that has several medical and pharmaceutical applications. The thermoplastic properties of EVA film make it suitable for laser microdissection14. For xMD, we initially used a 100-μm-thick DuPont ELVAX 410 EVA polymer extruded onto a paper-release liner as a thermoplastic seam tape (Electroseal) with an optimal heating temperature of 65 °C. However, this source has been discontinued and therefore we are currently using a CoTran membrane (3M, cat. no. 3M CoTran 9715). CoTran 9715 is a translucent EVA membrane containing 19% vinyl acetate; it has a thickness of 76.2 μm and an optimal heating temperature of 75 °C. Depending on the tissue type, target cell and specifics of the dissection process, different EVA films may be required to obtain satisfactory results. In general, the following features are optimal for xMD:
Film thickness 50–100 μm. Note that dissection of small targets may be facilitated with thinner films.
Optical translucency.
A concentration of vinyl acetate ~20%. The concentration of vinyl acetate correlates with melting temperature. In our tests, films with higher melting temperatures generally increase the specificity of microdissection.
Figure 2.
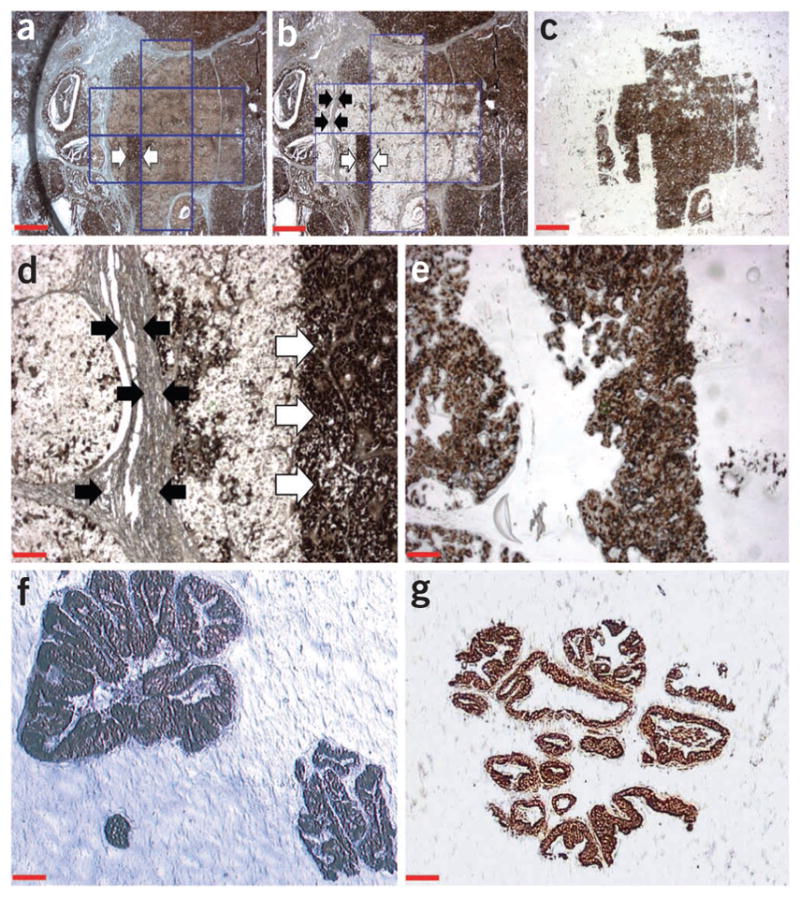
Examples of xMD using commercial microdissection instruments. (a) Low magnification view of a FFPE tissue section showing a high-grade breast carcinoma. The tumor cells are immunolabeled for estrogen receptors (brown nuclear staining) without counterstaining. The slide does not have a cover slip and the tissue is covered by EVA film. The area demarked by blue lines was irradiated using the xMD protocol with an ArcturusXT instrument. Arrows indicate a tumor area that was intentionally not irradiated by the laser. Magnification, ×20; scale bar, 500 μm. (b) The same breast carcinoma section after xMD and removal of the EVA film. Note that, within the irradiated area, only estrogen-positive cells were microdissected, whereas negative regions such as stroma and fibroblasts (demarcated by black arrows) were not captured and remain on the slide. Further, the tumor area that was not irradiated for xMD (white arrows) remains intact. Magnification, ×20; scale bar, 500 μm. (c) Low-magnification view of the same EVA film in panels a and b after xMD. The tumor cells positive for estrogen receptor are selectively captured onto the EVA film. However, the film did not capture the non-stained cells, stroma or the tumor cells within areas that were not irradiated. Magnification, ×20; scale bar, 500 μm. (d) Higher magnification view of the area of cancer cells (from panel b) positive for estrogen receptor that were captured using xMD. Note that negative stroma (black arrows) and cancer cells that were not irradiated (white arrows) remain on the slide. Magnification, ×100; scale bar, 100 μm. (e) EVA polymer from the same area shown in Figure 2d. Only IHC-positive cells were transferred. No stromal cells or unselected areas were captured. Magnification, ×100; scale bar, 100 μm. (f) Anti-cytokeratin AE1/AE3-stained EFPE prostate epithelial tissue that was transferred onto EVA film using the PixCell II instrument. Magnification, ×100; scale bar, 100 μm. (g) Anti-cytokeratin AE1/AE3-stained EFPE prostate epithelial tissue that was transferred onto EVA film using the Veritas instrument. Magnification, ×100; scale bar, 100 μm.
Laser parameters
For each laser source, various parameters were tested, including power levels and duration of the laser pulse. Overall, xMD can be performed with settings available in all tested instruments, usually requiring longer pulse duration times (between 15 and 50 ms) and a higher overlap (50%) of the pulses than in conventional laser dissection, with power settings dependent on the type of target cells (see PROCEDURE). In general, optimal laser parameters for xMD using LCM systems range from 30 to 75 mW. PixCell II and Veritas require manual focus adjustment of the IR laser, in conjunction with a Macro Cap stripped of its original film and placed on the slide for xMD. The ArcturusXT instrument does not have manual focus adjustment of the IR laser; however, the plastic cap is still necessary for successful dissection.
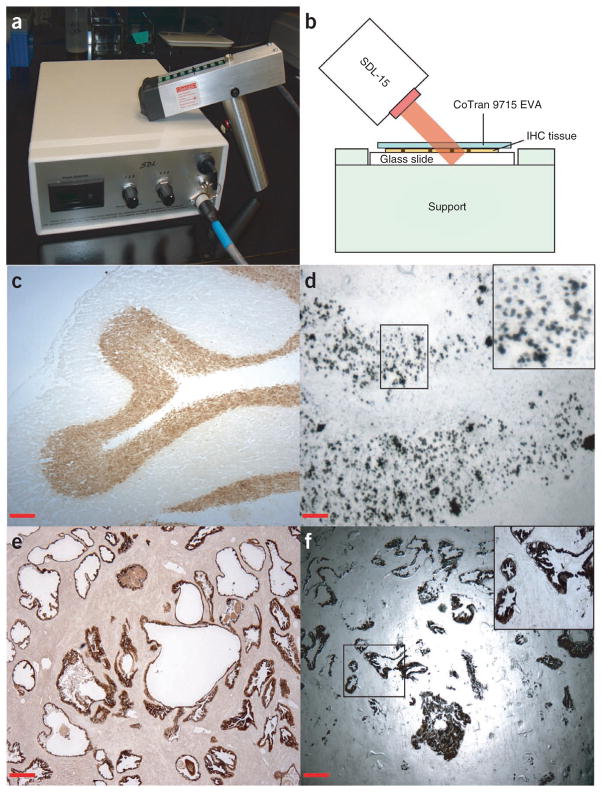
For the handheld laser device, we used a commercially available SDL-15 system from Biotechnique Avance. This is an 808 nm wavelength laser ‘gun’ adapted for xMD to heat darkly stained cells within the tissue (Fig. 3a,b). By using the premelting step described above to enhance the contact between the film and tissue, we recovered NeuN-stained individual nuclei from frozen rat brain tissue (Fig. 3c,d) and procured cytokeratin AE1/AE3-positive epithelium from EFPE prostate tissue (Fig. 3e,f). These experiments demonstrate the speed at which xMD dissections can be completed (10–15 min), as well as precision (nuclei). Moreover, the use of a relatively inexpensive laser system allows dissections to be performed for the first time in an instrument-free, ‘kit-like’ manner. In other words, the protocol can be performed on any laboratory bench by using xMD films and a low-cost light source. However, it should be noted that handheld xMD is the most recent iteration of the technology; thus, we have the least amount of experience with this version and investigators may need to adapt the protocol (laser pulse duration and power; film contact parameters) to meet their specific needs.
Figure 3.
Handheld xMD. (a) The SDL-15 laser diode device used for handheld xMD. The instrument allows for rapid microdissection of IHC-labeled cells in a relatively low-cost format. However, appropriate laser safety precautions must be used (see Box 4). (b) Schematic diagram showing the positioning of the SDL-15 laser diode for xMD. The slide is covered by EVA film (CoTran 9715 EVA) and placed on a support. The laser gun is pointed at an angle of 45° at a distance of 2–3 cm from the slide surface. (c) NeuN immunostaining of a frozen rat cerebellum section. Magnification, ×40; scale bar, 250 μm. (d) An EVA film after xMD procurement of NeuN-positive nuclei from frozen rat cerebellum. The nuclei of neurons from the granular layer were targeted using NeuN antibody and the entire tissue section was irradiated using the SDL-15 laser. IHC-positive individual nuclei were transferred to the EVA film demonstrating the high-resolution microdissection capability of xMD for certain applications. Magnification, ×100; scale bar, 100 μm. (e) Image of an anti-cytokeratin AE1/AE3-stained human prostate tissue section. Magnification, ×40; scale bar, 250 μm. (f) EVA film showing prostate epithelial cells procured by xMD using anti-cytokeratin AE1/AE3 targeting. An entire histological field of epithelial cells was microdissected in less than 10 min using the handheld laser. Magnification, ×40; scale bar, 250 μm.
Pilot tests and controls
At the outset of an xMD study, it is important to conduct pilot tests to evaluate the status of biomolecules in samples and to determine optimal dissection conditions, in much the same way as investigators do when using standard laser instruments. At the NIH LCM Core, we recommend assessing biomolecule quality in the tissue section after IHC as a first step, thereby evaluating the effects of both upstream tissue processing (specimen procurement, fixation and embedding) and the immunostaining procedure. The investigator simply scrapes a small amount of tissue from an IHC-stained slide and from a nonstained control section into extraction buffer and analyzes the content according to the assay(s) to be used in the study, evaluating whether the stained tissue is compatible with the analysis method and further determining the approximate number of cells needed. When there are multiple specimens and reasons to believe that there could be differences in biomolecule quality among them, such as a clinical study involving multiple institutions, a scrape test should be performed on all samples. Next, the specific conditions for xMD need to be determined, as optimal laser parameters are dependent on the type of tissue, the size of target cells or structures (smaller ones often require lower laser power) and the specific IHC staining pattern. Finally, downstream molecular analysis of the dissected cells should use established controls, e.g., those described previously in Nature Protocols by Erickson et al.8 for mRNA measurements.
Future directions
Looking forward, there are several aspects of xMD technology that are likely to be improved on as more members of the research community gain access to the technique. As one example, future replacement of colorimetric dyes with heat-absorbing nanoparticles is a promising approach to reduce the deleterious effects of immunolabeling, as this will simplify the IHC procedure by removing oxygen free radicals and stain deposition from the protocol. As a second example, the exploration of new EVA polymer films is an exciting area for further development, especially with respect to dissection precision. In this protocol, we used the specific types and sources of EVA film described; however, investigators should not feel constrained by these parameters, and may in fact be able to improve on them by using EVA (or other polymers) from alternative sources or with different properties (see Box 2). The extreme fidelity by which irradiated dyes activate overlying EVA suggests that improvements in dissection precision may be possible, perhaps to the level of small cellular organelles or even discrete molecular complexes, although the parallel use of histological pretreatment steps may be necessary to achieve these goals by altering the force needed to procure minute cellular elements. Certainly, the evolution of xMD technology to this level of resolution would be an exciting advancement, opening up many new lines of investigation of cells in histological sections.
MATERIALS
REAGENTS
Frozen, ethanol-fixed or formalin-fixed, paraffin-embedded (FFPE) tissue specimens sectioned at a thickness of 4–12 μm and mounted on glass slides ▲ CRITICAL Frozen tissue sections should be sectioned onto positively charged slides and stored at − 80 °C. ▲ CRITICAL Sections from FFPE tissue should be mounted on charged slides to prevent detachment during the heat-induced epitope retrieval (HIER) procedure that is required for many primary antibodies. ▲ CRITICAL Proper thickness of the section depends on the tissue and target cell types. For example, lymphoma may require thinner sections (4–5 μm) as lymphocytes are small, whereas brain tissue may require thick sections (μ12 μm), because neurons are relatively large. For epithelial cell dissections from organs such as breast or prostate, sections are typically cut at 8 μm. ! CAUTION When handling tissue specimens, you should always follow universal blood-borne pathogen safety procedures, including the use of a lab coat, gloves and safety glasses. Extra care should be taken with unfixed frozen samples.
Antigen retrieval reagents. For FFPE tissues, antigen retrieval is sometimes necessary to expose the epitope of the target protein for primary antibody binding. HIER using citrate buffer or target retrieval solution (Dako, cat. no. S1699) provides satisfactory results with the majority of primary antibodies. ▲ CRITICAL Some primary antibodies may require special antigen retrieval conditions, such as the use of citrate buffer (pH 6 or 9). Check the optimal conditions with the data sheet of the particular antibody to be used.
Primary antibodies for immunolabeling target cells for xMD. Reagents used to develop the current protocol included antibodies against cytokeratin AE1/AE3 for human epithelial cells (AE1/AE3, mouse monoclonal, dilution 1:50; Dako, cat. no. M3515), estrogen receptor for human breast cancer specimens (mouse monoclonal, dilution 1:50; Dako, cat. no. M7047) and NeuN for frozen rat brain tissue (mouse monoclonal, dilution 1:1,000; Millipore, cat. no. MAB377). ▲ CRITICAL xMD requires strong IHC staining; therefore, the user should empirically determine the optimal primary antibody dilution for each individual target of interest.
Antibody diluents with background reducer (Dako, cat. no. S3022)
Secondary antibody and DAB labeling reagents (Dako EnVision Plus kit, Dako, cat. no. K4007). ▲ CRITICAL Store your antibodies and DAB reagents at 4 °C until use.
DAB enhancer (Dako, cat. no. S1961)
PBS (1×)
Deionized (DI) water
Ethanol (95 and 100% (vol/vol), molecular grade; Sigma-Aldrich, cat. no. E7023) ! CAUTION Ethanol is toxic. Always use ethanol solution inside a fume hood with proper air extraction.
Xylenes (Sigma-Aldrich, cat. no. 247642) ! CAUTION Xylene vapor is harmful and toxic. Always use xylenes inside a fume hood with proper air extraction.
Molecular extraction buffer. The type of extraction buffer depends on the downstream analysis method. In general, the same buffers available for laser microdissection can be used for xMD samples (see Box 1).
QIAamp DNA Micro kit (Qiagen, cat. no. 51304)
PicoPure kit (Life Technologies, cat. no. KIT0204)
Novex Tris-glycine SDS sample buffer (2×; Life Technologies, cat. no. LC2676)
Total protein isolation kit (ITSI Biosciences, cat. no. K-0011)
DuPont ELVAX 410 EVA polymer (DuPont)
Thermoplastic seam tape (Electroseal)
Eppendorf themomixer (Eppendorf)
Eppendorf tube (1.5-ml; Eppendorf)
EQUIPMENT
Black & Decker Handy Steamer (Black & Decker, cat. no. HS1000) used for HIER. Other options include a pressure cooker or a microwave.
Adhesive pads (Post-it Notes, 3M)
Sealing roller (Bio-Rad, cat. no. MSR-0001)
Scissors, forceps and fresh razor blades.
Transparent ethylene vinyl acetate (EVA) film (3M CoTran 9715 EVA membrane, 3M or similar; see Box 2)
A standard optical microscope, such as the Olympus BX41 (Olympus), with ×4, × 10 and ×20 objectives used to verify IHC staining specificity and to evaluate EVA polymer after xMD.
Microscope-mounted digital camera (Olympus Q-color3 (Olympus) or similar)
Laser capture microdissection (LCM) Macro caps (Life Technologies, cat. no. LCM0212) ▲ CRITICAL The commercially available caps contain a dyed film that must be removed with forceps before xMD (see Box 3).
Hot plate or hybridization chamber, such as StatSpin ThermoBrite (StatSpin, product number TS01; see Box 2).
Laser instrument. Four options described here are as follows:
ArcturusXT laser microdissection instrument (Life Technologies)
Veritas laser microdissection instrument (Life Technologies)
PixCell II laser capture microdissection instrument (Life Technologies)
Handheld, laser diode system (SDL-15, Biotechnique Avance) ! CAUTION The SDL-15 laser system can be harmful and requires additional safety procedures to protect the operator and lab personnel (see Box 4).
PAP pen
Lens paper
BOX 3. PREPARATION OF A MACRO LCM CAP FOR PERFORMING xMD.
When performing xMD using the ArcturusXT, Veritas or PixCell II systems, the laser must reach the proper focal plane at the surface of the tissue. This is achieved with optical plastic backing of a Macro LCM cap (Life Technologies). However, commercially available LCM caps contain a dyed film that must be removed before xMD. The following simple procedure is used:
While you are wearing gloves, carefully remove a cap from its plastic tray.
With cleaned forceps, pull the plastic film from the cap.
Clean the plastic surface of the cap with lens paper soaked in 70% (vol/vol) ethanol.
Carefully replace the cap in the original plastic tray.
Note: the prepared cap can be reused during the xMD procedure.
BOX 4. LASER SAFETY PRECAUTIONS.
All the commercially available laser dissection instruments described here (ArcturusXT, Veritas, PixCell II) have enclosed lasers; therefore, no additional safety precautions are necessary for the expression microdissection (xMD) protocol. However, the handheld xMD device is an ‘open’ laser source and thus requires the following safety measures:
All users must wear appropriate-wavelength laser safety goggles with an optical density (OD) value higher than 7 in the 808-nm range.
All users should also wear lab coats and latex gloves.
The laser should be housed in a lockable room with appropriate ‘warning’ signage.
All windows and other openings of the room must be covered to prevent accidental exposure to individuals.
An acrylic box structure should be used to house the laser and the slide to prevent accidental exposure. One example is a laser safety dome by Laservision (cat. no. 1307, dome filter pink (OD > 7 at 755–830 nm)).
The work area should be free of reflective surfaces that could cause accidental exposure.
Before you use the handheld laser device, a laser safety representative should evaluate the work environment.
PROCEDURE
Slide preparation for xMD ● TIMING 2 h 20 min to 1 d (depending on incubation time)
-
1|
Stain the slide by IHC using a primary antibody of choice. An example IHC protocol is provided in Box 5.
! CAUTION All animal tissues must be obtained according to relevant guidelines and regulations. All human tissue samples must be obtained according to institutional review board protocols, including informed consent from subjects.
▲ CRITICAL STEP Do not counterstain the tissue with hematoxylin or other stains following IHC. Do not place a cover slip on the stained tissue section.
▲ CRITICAL STEP If RNA or proteins are to be extracted, we recommend performing xMD immediately after IHC staining.
■ PAUSE POINT If DNA extraction is to be performed, the stained slides can be stored in a tightly closed jar containing desiccant, avoiding direct light exposure. The slides can be used safely within 1 week.
? TROUBLESHOOTING
-
2|
With a razor blade, cut a section of EVA film that is sufficient to cover the tissue section, but that is slightly narrower than the width of the slide. This is important to ensure that EVA on the slide fits in the slide holder of the automated instruments.
-
3|
With forceps, you should carefully place the trimmed EVA polymer on the tissue section.
-
4|
Apply the sealing roller with uniform pressure across the EVA film on the slide.
▲ CRITICAL STEP This is necessary to ensure good contact between the EVA film and the tissue.
-
5|
Place the slide with the EVA film on a hot plate or into a hybridization chamber set at 60–80 °C for 2–5 min.
▲ CRITICAL STEP The optimal heating temperature needs to be predetermined by the user and depends on the type of EVA film used (see Box 2).
? TROUBLESHOOTING
-
6|
Apply the roller to the EVA polymer surface using gentle and uniform pressure.
▲ CRITICAL STEP Because the EVA film is warm and malleable, contact between the film and the tissue is further improved.
? TROUBLESHOOTING
-
7|
Allow the slide with the EVA film to cool at room temperature (20–25 °C) for 5 min. The slide is now ready for xMD.
▲ CRITICAL STEP Once the EVA film has adhered to the slide, it is our experience that it is best to proceed immediately to xMD.
? TROUBLESHOOTING
BOX 5. IMMUNOHISTOCHEMISTRY FOR xMD.
Performing xMD requires the use of a chromogen such as 3,3′-diaminobenzidine (DAB) that absorbs the laser energy and activates the EVA film adjacent to the target cells. A typical IHC staining protocol follows these general steps:
For FFPE tissue, remove the paraffin wax from the histological section with a xylene bath and hydrate the tissue with decreasing grades of ethanol (100%, 95% and 70% (vol/vol)) and then with DI water.
Once the tissue is hydrated, proceed with an antigen retrieval step if necessary. For HIER, use 1× Dako target retrieval solution preheated for 20 min in a Black & Decker steamer. Carefully place the slides inside the heated target retrieval solution for 30 min. Let the slides cool down to room temperature (RT) and transfer them to DI water.
If frozen sections are used, thaw the slide immediately before staining and place the section in 70% (vol/vol) ethanol for 1 min. Transfer the slide to DI water.
Use a PAP pen to demark a thin hydrophobic barrier around the tissue to concentrate the incubation solution over the tissue area. Do not use an excess of PAP pen, as it may spread onto the tissue and compromise IHC staining.
Using the Dako Envision Plus kit, apply peroxidase blocking solution (bottle 1 in the kit) for 10 min. Cover the tissue generously with solution. The incubation time may need to be adjusted, as each tissue type contains different levels of endogenous peroxidase activity.
Wash the slide in a DI water bath.
Transfer the slide to 1× PBS.
Apply primary antibody over the tissue section. The antibody titer, incubation time and incubation temperature depend on the specific antibody and antigen abundance. Refer to the data sheet provided by the manufacturer as a starting point.
Wash the slide in 1× PBS (30 s, three times).
Using the Dako EnVision Plus kit, apply the secondary antibody (bottle 2) for 30 min at RT. Be aware that the primary antibody may be monoclonal (mouse) or polyclonal (rabbit); hence, verify that the appropriate secondary antibody is used.
Wash the slide in 1× PBS (30 s, three times).
Prepare the DAB solution (one drop of DAB in 1 ml of DAB substrate solution; bottle 3).
Apply the DAB solution on the slide for 5 min at RT. The user should monitor this step carefully, as sufficient staining may be achieved before 5 min.
Wash the tissue in DI water bath (30 s, two times).
Apply Dako DAB enhancer on the slide for 3 min at RT.
Wash in DI water bath.
Place the slide in 70% (vol/vol) ethanol bath.
Place the slide in 95% (vol/vol) ethanol bath for 2 min (two times).
Place the slide in 100% ethanol bath for 2 min (two times).
Place the slide in xylene for 3 min.
Let the slide air dry in a fume hood. Do not apply counterstaining or a cover slip. The slide is now ready for xMD.
xMD process ● TIMING 10–60 min per slide, depending on the laser system used
-
8|
xMD can be performed using option A (ArcturusXT), option B (Veritas) or option C (Pixcell II) as described below. Furthermore, the protocol can be performed using option D, a handheld laser system that does not require a laser micro-dissection instrument.
? TROUBLESHOOTING
(A) xMD with the ArcturusXT microdissection system
Place the prepared slide with EVA film on the slide holder of the instrument.
Load the modified xMD caps, which lack the original dyed film (see Box 3).
With the instrument controls, place the cap on an area of the slide containing IHC-positive cells.
-
Using the ArcturusXT capture laser control, you should set up the system according to the following parameters: spot size 50 μm, laser pulse duration between 30 and 50 ms, laser power between 30 and 75 mW, and 50% spot overlap.
▲ CRITICAL STEP The optimal laser parameters depend on the type of tissue, target cell and IHC marker. In general, finer targets require smaller spot sizes, lower laser power and less duration than larger targets such as large epithelial cells; however, preliminary tests are needed to find the optimal laser parameters for each particular application.
-
Using the ArcturusXT control software, you should select the region to be irradiated by the capture laser, including IHC-positive cells, and then apply the command for capture.
▲ CRITICAL STEP The whole field or the whole area under the cap, including both positive and negative cells, can be selected. xMD will only capture IHC-positive cells.
▲ CRITICAL STEP Do not use the cutting laser at any point in the xMD procedure.
If another area of the stained tissue is to be microdissected, using the instrument controls, you must select the new area under the microscope and place the same cap under the new region center. Repeat Step 8A(v).
Repeat the process until all areas of IHC-positive cells are dissected.
Once finished, remove the cap from the slide by moving the cap to the quality control position. Thereafter, remove the slide with the film by clicking on the < present stage > button in the ArcturusXT control panel.
(B) xMD with the Veritas microdissection system
Using the Veritas control panel, you should open the instrument and load the prepared slide with the EVA film onto the slide holder.
Load the modified xMD caps (see Box 3) into the cap holder.
The Veritas instrument will automatically take a ‘road map’ image of the loaded slide. Thereafter, with the Veritas control panel, place a cap on an area of the slide containing IHC-positive cells.
Using the Veritas microscope control panel, you should select an area under the cap with a clear space for laser focus adjustment.
With the microscope control panel, select the ×10 objective.
Check that the field is still on the area with a clear space under the cap. Then, with the Veritas control panel, turn down the light intensity.
-
Using the capture laser control panel, you should locate and focus the capture laser.
▲ CRITICAL STEP The Veritas instrument requires user-dependent focusing of the capture laser before xMD.
▲ CRITICAL STEP Do not use the cutting laser at any point in the xMD procedure.
-
With the capture laser controls, set up the IR laser according to the following parameters: spot size 50 μm, laser pulse duration between 3 and 10 ms, laser power between 45 and 75 mW, and 50% spot overlap.
▲ CRITICAL STEP Optimal laser parameters depend on the type of tissue, target cell and IHC marker. In general, finer targets require smaller spot size, lower laser power and less duration than larger targets such as large epithelial cells; however, preliminary tests are needed to find the optimal laser parameters for each particular application.
-
In the Veritas control software, select the region to be irradiated by capture laser and then apply the command for capture.
▲ CRITICAL STEP The whole field or the area under the cap, including both positive and negative cells, can be selected. xMD will capture only IHC-positive cells. To avoid errors in the Veritas software, do not select a single area larger than the ×10 field of view on the Veritas. Multiple ×10 fields are acceptable.
If there is another area of the tissue with IHC-positive cells to be microdissected, with the instrument controls, select the new area under the microscope and place the same cap under the new region. Repeat Step 8B(ix).
Once you are finished, remove the cap from the slide by moving the cap to quality control position. Then, with the control panel, open the Veritas door and remove the slide with the EVA film from the Veritas.
(C) xMD with the PixCell II laser capture microdissection instrument
Place the prepared slide with EVA film on the microscope platform of PixCell II.
Place the modified xMD caps (see Box 3) on the microscope cap tray.
While looking at the screen or through the microscope eyepieces, you should select an area with IHC-positive cells.
Activate the vacuum control in the PixCell II control box.
-
Using the metallic arm with the cap holder, you should remove one cap and place it on the selected area of the slide. (vi) Gently press the metallic cap holder against the slide manually.
▲ CRITICAL STEP This step improves the contact of the film with the tissue section.
Activate the laser control in the PixCell II control box.
-
Using the screen, you should find a clear area under the cap. Move the microscope to the ×10 objective and reduce the amount of light to focus the laser.
▲ CRITICAL STEP The xMD procedure on the PixCell II requires fine laser focus.
-
With the PixCell II control box, adjust the laser according to the following parameters: laser power, between 30 and 100 mW; pulse duration, 15–50 ms; repeat t = 0.2 s; target = 0.300 V; spot size = 7.5 and 30 μm.
▲ CRITICAL STEP The optimal laser parameters depend on the type of tissue, target cell and IHC marker. In general, fine targets require smaller spot size, lower laser power and less duration than do larger targets such as large epithelial cells; however, preliminary tests are needed to find the optimal laser parameters for each particular application.
-
Irradiate the entire area under the cap, including IHC-stained and unstained cells.
▲ CRITICAL STEP Only IHC-positive cells are captured.
If another area of the tissue also contains IHC-positive cells, move the cap to the new area and repeat the previous steps.
Once you are finished, remove the cap from the slide using the metallic arm. Then, remove the slide with the EVA film from the microscope platform.
(D) xMD procedure with a handheld laser
-
This option does not require the use of a laser microdissection instrument. Instead, a handheld laser such as the SDL-15 diode is used as a laser source. Place the prepared slide with the EVA inside a safety box (see Box 4).
! CAUTION Wear a lab coat, gloves and laser safety glasses (see Box 4).
Set the SDL-15 instrument to the number three (‘3’) position for pulse frequency (7 pulses per s) and to the number two (‘2’) setting for laser intensity (5.7 J cm2).
-
Hold the SDL-15 laser gun at a 45° angle to the slide surface and keep it between 2 and 3 cm above the surface of the EVA film.
▲ CRITICAL STEP Do not allow the tip of the laser gun to contact the surface of the film, as this may damage the system and compromise dissection.
-
Irradiate the tissue covered by EVA film with ~1,200 laser pulses.
▲ CRITICAL STEP The number of laser pulses depends on the size of the tissue to be irradiated.
▲ CRITICAL STEP When irradiating the EVA, a wetting appearance is observed when the film is successfully activated.
Removal and incubation of EVA film after xMD ● TIMING 10 min or until molecular extraction process is complete
-
9|
After completing xMD following any of the previous options, you should place the slide with EVA film on the bench.
-
10|
Remove the EVA with forceps from the histological slide in one rapid motion.
-
11|
Place the EVA film on a new glass slide (without any tissue) to verify the presence of procured IHC-positive cells using a standard microscope.
-
12|
If tissue debris is detected on the EVA polymer, apply an adhesive pad to the EVA to remove any unwanted material.
▲ CRITICAL STEP In general, xMD is very specific for target cells; however, occasionally, you will observe the presence of off-target debris, particularly when the tissue is excessively dried after staining, when folds are present in the tissue section or in the presence of cellular necrosis. Similar to standard laser dissection, off-target debris can be removed with the application of the adhesive side of a sticky note pad (e.g., Post-it note)8.
? TROUBLESHOOTING
-
13|
Once the presence of IHC-positive cells transferred onto the EVA film has been confirmed, cut the film with a razor blade and place the pieces inside a 1.5-ml Eppendorf tube containing the proper extraction buffer in a sufficient amount to cover the pieces of film. Check the manufacturer’s instructions regarding the proper incubation time (see Box 1).
■ PAUSE POINT The molecular extraction procedure is performed using standard protocols for LCM samples. A number of downstream analysis methods can be employed with the extracted biomolecules, including DNA methylation analysis12,13,22; proteomics26 and RT-PCR27. Depending on the molecule and the kit or protocol employed, the cell extract can be temporarily stored. For example, the PicoPure™ RNA kit (Life Technologies) allows the extracted sample to be stored at − 80° C until use.
? TROUBLESHOOTING
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1 | Cells did not transfer to EVA | The slide was not properly dehydrated | Increase the length of time in the final 100% ethanol and xylene baths (Box 5) Use fresh ethanol solutions |
| 5–7 | Cells did not transfer to EVA | EVA was not properly prepared | Increase the time of heat incubation of the EVA film Apply the roller on the EVA with more pressure |
| 8 (options A–D) | Cells did not transfer to EVA | Laser setup not adequate | Test a different laser setup: increase power and/or duration |
| 8 (options B, C) | Cells did not transfer to EVA | Laser was not properly focused | Focus the capture laser before xMD |
| 12 | Too much tissue debris on the film | Slide too dry | Decrease the length of time in the final 100% ethanol and xylene baths (Box 5) Apply an adhesive pad on the EVA with gentle pressure to remove the tissue debris |
| 13 | No biomolecules detectable after extraction | Buffer did not cover the film | Increase the amount of extraction buffer to cover all the pieces of the film Use a shaker or themomixer for incubation Test a different extraction buffer |
● TIMING
Step 1, Including Box 5: 2 h to overnight (depending on the incubation time needed for the primary antibody).
Steps 2–7: 15–20 min
Step 8A, ArcturusXT: 30–45 min, depending on the type of tissue and target cells
Step 8B, Veritas: 30–45 min, depending on the types of tissue and target cells
Step 8C, PixCell II: 45–60 min, depending on the types of tissue and target cell
Step 8D, handheld xMD: 10–15 min, depending on the size of the tissue section
Steps 9–12: 10 min
Step 13: variable, dependent on the type of extraction buffer (see Box 1)
ANTICIPATED RESULTS
Typical xMD results obtained with each of the laser systems are shown in Figures 2 and 3. After a successful dissection, the xMD film should contain the IHC-positive cells captured on the film without significant contamination by IHC-negative cells or tissue debris. The results of the dissection can be evaluated using a light microscope.
The yield of biomolecules depends on the number of procured cells and on the method of tissue preservation. However, biomolecule recovery can be negatively affected by the IHC staining process; thus, it is important to analyze an IHC-stained tissue section at the outset of a study to obtain an estimate of quality and quantity. In addition, spectrophotometer-based measurement (NanoDrop, Thermo Scientific) of the extracted biomolecules is recommended.
Acknowledgments
This research was supported, in part, by the intramural program of the NIH National Cancer Institute, Center for Cancer Research.
Footnotes
AUTHOR CONTRIBUTIONS R.F.B., T.J.P., M.A.T. and M.R.E.-B. developed expression microdissection (xMD). J.C.H., M.A.T. and M.R.E.-B. designed the experiments. J.C.H., S.K., M.D.A. and M.A.T. conducted the tests. J.C.H., J.R.-C., M.A.T. and M.R.E.-B. analyzed the data. J.C.H., M.A.T., J.R.-C. and M.R.E.-B. wrote the manuscript. J.C.H. and M.A.T. contributed equally to the work.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests (see the HTML version of this article for details).
References
- 1.Emmert-Buck MR, et al. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 2.Bonner RF, et al. Laser capture microdissection: molecular analysis of tissue. Science. 1997;278:1481–1483. doi: 10.1126/science.278.5342.1481. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JL, Finkelstein SD. Microdissection techniques for molecular testing in surgical pathology. Arch Pathol Lab Med. 2004;128:1372–1378. doi: 10.5858/2004-128-1372-MTFMTI. [DOI] [PubMed] [Google Scholar]
- 4.Espina V, et al. Laser-capture microdissection. Nat Protoc. 2006;1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 5.Dupont Jensen J, et al. PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res. 2010 Oct 12; doi: 10.1158/1078-0432.CCR-10-1133. published online. [DOI] [PubMed] [Google Scholar]
- 6.Pinós T, et al. A novel mutation in the mitochondrial tRNA(Ala) gene (m. 5636T > C) in a patient with progressive external ophthalmoplegia. Mitochondrion. 2011;11:228–233. doi: 10.1016/j.mito.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, et al. Stromal microenvironment processes unveiled by biological component analysis of gene expression in xenograft tumor models. BMC Bioinformatics. 2010;11(Suppl 9):S11. doi: 10.1186/1471-2105-11-S9-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson HS, et al. Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat Protoc. 2009;4:902–922. doi: 10.1038/nprot.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johann DJ, et al. Approaching solid tumor heterogeneity on a cellular basis by tissue proteomics using laser capture microdissection and biological mass spectrometry. J Proteome Res. 2009;8:2310–2318. doi: 10.1021/pr8009403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein CJ, et al. Mass spectrometric-based proteomic analysis of amyloid neuropathy type in nerve tissue. Arch Neurol. 2010 Oct 11; doi: 10.1001/archneurol.2010.261. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangrea MA, et al. Expression microdissection: operator-independent retrieval of cells for molecular profiling. Diagn Mol Pathol. 2004;13:207–212. doi: 10.1097/01.pdm.0000135964.31459.bb. [DOI] [PubMed] [Google Scholar]
- 12.Hanson JA, et al. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98:255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 13.Grover AC, et al. Tumor-associated endothelial cells display GSTP1 and RARbeta2 promoter methylation in human prostate cancer. J Transl Med. 2006;4:13. doi: 10.1186/1479-5876-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein SR, McQueen PG, Bonner RF. Thermal modeling of laser capture microdissection. Appl Opt. 1998;37:7378–7391. doi: 10.1364/ao.37.007378. [DOI] [PubMed] [Google Scholar]
- 15.Perlmutter MA, et al. Comparison of snap freezing versus ethanol fixation for gene expression profiling of tissue specimens. J Mol Diagn. 2004;6:371–377. doi: 10.1016/S1525-1578(10)60534-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leiva IM, Emmert-Buck MR, Gillespie JW. Handling of clinical tissue specimens for molecular profiling studies. Curr Issues Mol Biol. 2003;5:27–35. [PubMed] [Google Scholar]
- 17.Fend F, et al. Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–66. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Canales J, et al. Identification of a unique epigenetic sub-microenvironment in prostate cancer. J Pathol. 2007;211:410–419. doi: 10.1002/path.2133. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald JA, Murugesan N, Pachter JS. Validation of immuno-laser capture microdissection coupled with quantitative RT-PCR to probe blood-brain barrier gene expression in situ. J Neurosci Methods. 2008;174:219–226. doi: 10.1016/j.jneumeth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Immuno-laser capture microdissection of frozen prolactioma sections to prepare proteomic samples. Colloids Surf B Biointerfaces. 2009;71:187–193. doi: 10.1016/j.colsurfb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Fend F, Kremer M, Quintanilla-Martinez L. Laser capture microdissection: methodical aspects and applications with emphasis on immuno-laser capture microdissection. Pathobiology. 2000;68:209–214. doi: 10.1159/000055925. [DOI] [PubMed] [Google Scholar]
- 22.Eberle FC, et al. Immunoguided laser assisted microdissection techniques for DNA methylation analysis of archival tissue specimens. J Mol Diagn. 2010;12:394–401. doi: 10.2353/jmoldx.2010.090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckanovich RJ, et al. Use of immuno-LCM to identify the in situ expression profile of cellular constituents of the tumor microenvironment. Cancer Biol Ther. 2006;5:635–642. doi: 10.4161/cbt.5.6.2676. [DOI] [PubMed] [Google Scholar]
- 24.von Smolinski D, Blessenohl M, Neubauer C, Kalies K, Gebert A. Validation of a novel ultra-short immunolabeling method for high-quality mRNA preservation in laser microdissection and real-time reverse transcriptase-polymerase chain reaction. J Mol Diagn. 2006;8:246–253. doi: 10.2353/jmoldx.2006.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown AL, Smith DW. Improved RNA preservation for immunolabeling and laser microdissection. RNA. 2009;15:2364–2374. doi: 10.1261/rna.1733509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha S, et al. In situ proteomic analysis of human breast cancer epithelial cells using laser capture microdissection: annotation by protein set enrichment analysis and gene ontology. Mol Cell Proteomics. 2010;9:2529–2544. doi: 10.1074/mcp.M110.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nonn L, Vaishnav A, Gallagher L, Gann PH. mRNA and micro-RNA expression analysis in laser-capture microdissected prostate biopsies: valuable tool for risk assessment and prevention trials. Exp Mol Pathol. 2010;88:45–51. doi: 10.1016/j.yexmp.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]