Abstract
Study Objectives:
This study aimed to examine the effect of sleep state (rapid eye movement [REM] versus non-rapid eye movement [NREM]) and position (supine versus non-supine position) on obstructive respiratory events distribution in adolescent population (ages 12 to 18 y).
Methods:
This was a retrospective study that included 150 subjects between the ages of 12 to 18 y with an apnea-hypopnea index (AHI) > 1/h. Subjects using REM sleep–suppressant medications and subjects with history of genetic anomalies or craniofacial syndromes were excluded.
Results:
The median age was 14 y with interquartile range (IQR) of 13 to 16 y, 56% of patients were males and the median body mass index (BMI) z-score was 2.35 (IQR: 1.71–2.59) with 77.3% of patients fulfilling obesity criteria. Respiratory obstructive events were more common in REM sleep. The median REM obstructive AHI (OAHI) was 8.9 events per hour (IQR: 2.74–22.8), whereas the median NREM OAHI was 3.2 events per hour (IQR: 1.44–8.29; p < 0.001). African American adolescents had more REM obstructive events with median REM OAHI of 13.2 events per hour (IQR: 4.88–30.6), which was significantly higher than median REM OAHI of 4.94 (IQR: 2.05–11.36; p = 0.004) in white adolescents. Obstructive events were more common in supine position with higher median supine OAHI of 6.55 (IQR: 4–17.73) when compared to median non-supine OAHI of 2.94 (IQR: 1–6.54; p < 0.001).
Conclusions:
This study shows that sleep related obstructive respiratory events in the adolescents (12 to 18 y of age) occur predominantly in REM sleep and in supine position.
Citation:
El-Kersh K, Cavallazzi R, Patel PM, Senthilvel E. Effect of sleep state and position on obstructive respiratory events distribution in adolescent children. J Clin Sleep Med 2016;12(4):513–517.
Keywords: adolescent sleep apnea, NREM OSA, REM OSA
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep related breathing disorder that can affect up to 5% of children.1 It is characterized by partial or complete upper airway obstruction that can disrupt normal sleep ventilation and sleep pattern.2 This upper airway obstruction can occur in rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. In REM sleep, the withdrawal of excitatory noradrenergic and serotonergic inputs to upper airway motor neurons further reduces pharyngeal muscle activity, which together with reduced arousal threshold and reduced ventilatory responses to hypoxia and hypercapnia increase the propensity for upper airway obstruction in REM sleep, with more prolonged obstructions accompanied by severe desaturations.3
Although obstructive respiratory events in children, unlike adults, is thought to be REM predominant, a subset of pediatric population may have NREM predominance of their obstructive respiratory events.4–6 Also, the effect of position on the distribution of obstructive respiratory events in the pediatric population is not consistent among different studies.7–12 Furthermore, previous studies assessing the effect of sleep stage and position on the distribution of obstructive respiratory events did not explore such effects in a pediatric adolescent population.4–12
BRIEF SUMMARY
Current Knowledge/Study Rationale: The effect of sleep state and position on the distribution of obstructive respiratory events was previously described in young pediatric population. However, there is a paucity of data on this effect in adolescents.
Study Impact: Our study describe the effect of sleep state and position on the distribution of obstructive respiratory events in adolescent population. Our findings help to better understand sleep related breathing disorders in adolescents.
Objective
The objective of this study was to examine the distribution of obstructive respiratory events in REM versus NREM sleep, and in supine versus non-supine sleep positions in an adolescent population (ages 12 to 18 y).
METHODS
This was a retrospective study approved by the university Institutional Review Board. Informed consent was waived.
Study Population
Nocturnal polysomnograms (PSGs) of 285 children between ages 12 and 18 y who were referred to the University of Louisville Pediatric Sleep Center over a 4-y period (January 2008–July 2012) for possible sleep disordered breathing were retrospectively analyzed.
Patients between the ages of 12 to 18 y with an apnea-hypopnea index (AHI) > 1/h were included in the study. Exclusion criteria included subjects with genetic anomalies or cranio-facial syndromes and current use of REM sleep–suppressant medications.
Data collection from the patient chart and PSGs included age, race, sex, body mass index (BMI), current use of REM sleep–suppressant medications, past medical history, history of adenotonsillectomy, total AHI, REM AHI, NREM AHI, obstructive apnea-hypopnea index (OAHI), REM OAHI, NREM OAHI, supine OAHI, non-supine OAHI, and oxyhemoglobin saturation nadir in both REM and NREM sleep. OAHI included obstructive apneas and hypopneas and mixed apneas.
Polysomnography
All included overnight PSGs were obtained at the University of Louisville Pediatric Sleep Medicine Center using a Stellate Harmonie-S Sleep System (Canada). All children were accompanied by one of their caregivers who slept in the same room (bed sharing was not allowed) during the sleep study. Polysomnography included eight channels of electroencephalogram (EEG), right and left electro-oculograms, submental and anterior tibial electromyogram, and electrocardiogram (EKG). Respiratory parameters were measured via thoracic and abdominal inductance plethysmography belts. Oronasal air flow was measured using a combined oronasal thermistor and nasal pressure transducer cannula. Pulse oximeter, end-tidal and transcutaneous carbon dioxide sensors, and snore microphone were utilized. Each sleep study was fully attended by a sleep technician and fully recorded via an overnight infrared camera.
Body Position Assessment
Patients were allowed to assume spontaneous body positions during their sleep. Sleep positions were categorized into supine or non-supine positions based on torso position. Body position was identified primarily by the position sensor, situated on the thoracic belt.
Polysomnography Scoring
All polysomnography data were recorded to a computerized system and scored manually in 30-sec epochs (sleep and arousals), then 2-min epochs (respiratory events and periodic leg movements). All PSGs were scored by a registered sleep technologist and subsequently reviewed by an experienced pediatric sleep medicine physician. Sleep stages and respiratory events were scored according to the standard pediatrics criteria of American Association of Sleep Medicine (AASM) guidelines using the 2007 AASM scoring manual.13 Obstructive apneas were defined as the absence of airflow with continued increased inspiratory effort for duration of at least two breaths. Hypopneas were defined as a decrease in nasal pressure signal of ≥ 50% for duration of at least two breaths with a corresponding decrease in saturation of peripheral oxygen (SpO2) of 3% or more and/or EEG arousal.
Statistical Analysis
Data were analyzed using commercially available Stata 10 statistical software package (Stata Corp, College Station, TX). We present continuous variables as median and interquartile range (IQR), and categorical variables as numbers and percentages. We compared continuous variables with the Wilcoxon signed-rank test. We used linear regression model to evaluate the predictive effect of ethnicity and BMI z-score on REM OAHI. We also performed simple logistic regression to evaluate if the variables age, arousal index, and oxygen level nadir are associated with NREM predominance. For linear regression, we present the coefficient estimate and the corresponding 95% confidence interval. We present the results of the regression model as odds ratio and 95% confidence interval. For all analyses, we considered a value of p < 0.05 statistically significant.
RESULTS
A total of 150 subjects who successfully met the inclusion criteria, with no exclusion criteria, were included in the analysis. The median age was 14 y (IQR: 13–16), and 56% of subjects were males. Obesity was defined as BMI z-score > 1.65 (95th percentile) with 77.3% of subjects (n = 116) fulfilling obesity criteria. Other demographics and polysomnographic data are presented in Tables 1 and 2.
Table 1.
Patients' demographics.
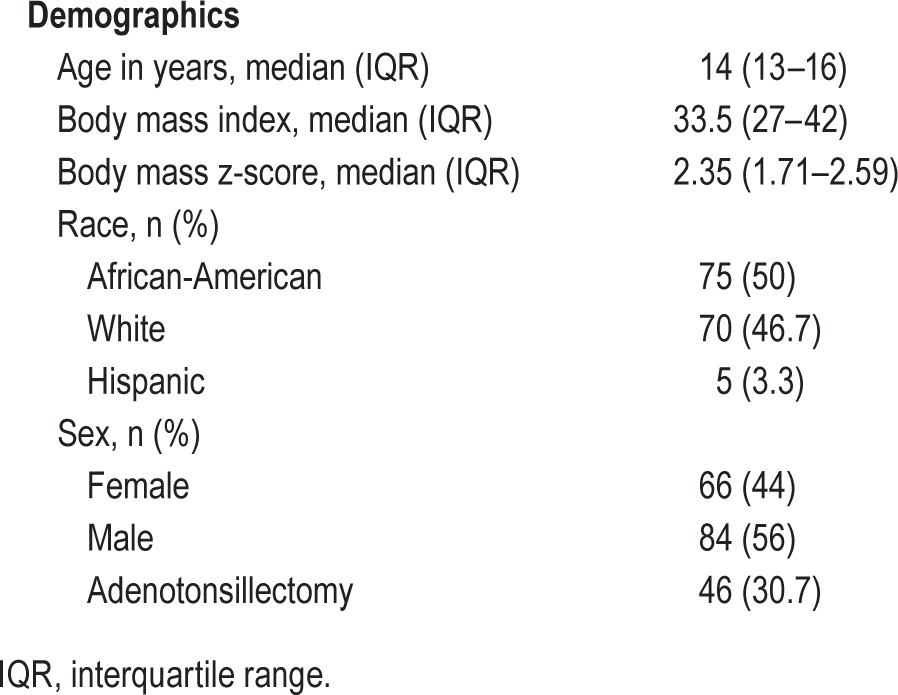
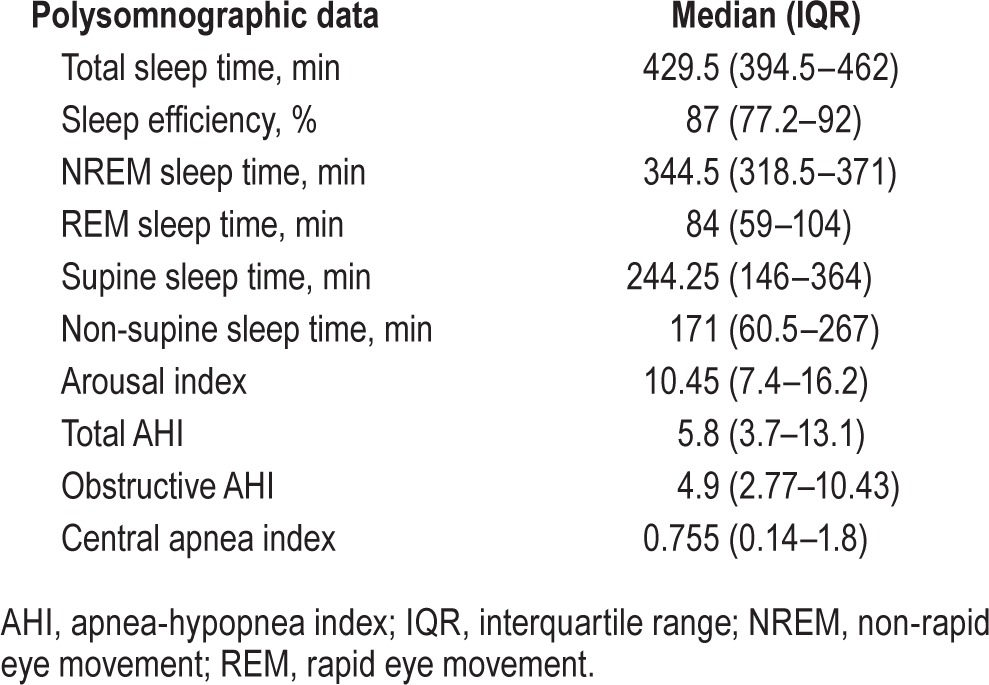
Table 2.
Polysomnographic data of the patients.
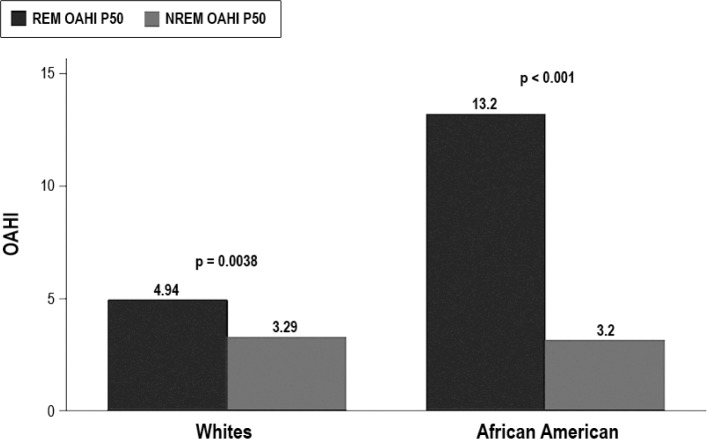
Overall, the median REM AHI was 10.64 (IQR: 4.86–25) and the NREM AHI was 4.23 (IQR: 2.3–10.9; p < 0.0001). Obstructive events were more common in REM sleep when compared to NREM sleep. The median REM OAHI was 8.9 events/h (IQR: 2.74–22.8), whereas the median NREM OAHI was 3.2 events/h (IQR: 1.44–8.29; p < 0.001). African American adolescents had more REM obstructive events with median REM OAHI of 13.2 events/h (IQR: 4.88–30.6) significantly higher than median REM OAHI of 4.94 (IQR: 2.05–11.36; p = 0.004) in white adolescents, but there was no difference in NREM OAHI (Figure 1). After adjustment for BMI z-score via linear regression analysis, being an African American remained a significant risk factor for higher REM OAHI with an increase of OAHI by 10.7 (95% confidence interval [CI]: 1.9–19.5; p = 0.017). There was no sex difference in REM obstructive events with a median male REM OAHI of 8.57 (IQR: 2.54–22.86) and a median female REM OAHI of 9.01 (IQR 2.89 –21.90; p = 0.94).
Figure 1. Median rapid eye movement obstructive apnea-hypopnea index.
Median REM OAHI (13.2) in African American adolescents was statistically significant higher than median REM OAHI (4.94) in white adolescents (p = 0.004). In both African American and white adolescents REM OAHI remained higher than NREM OAHI. REM, rapid eye movement; OAHI, obstructive apnea-hypopnea index; NREM, non-rapid eye movement.
Obstructive events were more common in the supine position. The median supine OAHI of 6.55 (IQR: 4–17.73) was significantly higher than non-supine median OAHI of 2.94 (IQR: 1–6.54; p < 0.001). Oxygen saturation nadir did not differ between REM sleep (median of 89.95%, IQR: 85.9–92) and NREM sleep (median of 89%, IQR: 85.9–91; p = 0.11).
Thirty-eight patients (25.3%) had more obstructive events during NREM sleep. We evaluated factors that may predict NREM predominance of obstructive events reported by Verginis et al.,5 namely age, higher arousal index, and oxygen level nadir.5 Age (odds ratio [OR] = 0.99; 95% CI 0.81–1.21; p = 0.97), arousal index (OR = 1.03; 95% CI 0.98–1.08; p = 0.29), and oxygen level nadir (OR = 1.05; 95% CI 0.97–1.14; p = 0.18) were not significant risk factors for NREM predominance of obstructive events in our cohort.
DISCUSSION
The main finding of our study is that adolescent population has more obstructive respiratory events during REM sleep and in supine position. However, a substantial proportion of patients (25.3%) had a predominance of obstructive events during NREM sleep.
In adults with OSA sleep fragmentation tends to be pronounced, and the respiratory events are NREM predominant, but up to one third of sleep disordered breathing can occur during REM sleep.14,15 In children with OSA, the opposite is true. Sleep architecture is usually preserved, and most of the respiratory events are REM predominant, but up to one third of children population with moderate to severe OSA have predominant respiratory events during NREM sleep. These NREM events tend to be associated with more arousals but less desaturations.4,5
At birth, the full-term infant has equal amounts of REM and NREM sleep. REM sleep time decreases with age to occupy almost 25% of the total sleep time, as in an adult person, by age 3 to 5 y.13 Studies suggested the importance of REM sleep in learning and memory consolidation.16,17 Because REM apneas tend to be longer and associated with longer desaturations, REM-predominant apneas may predispose the exposed children population to higher risk of future neurocognitive dysfunction.5,9,18
Prior studies that evaluated sleep distribution of respiratory events between REM and NREM sleep in children did not target adolescent population. In fact, the mean age of the enrolled subjects in these studies ranged between 5 to 7.3 y, which is much younger than the mean age (14.3 y) of the adolescent subjects included in this study.4–6
In our adolescent cohort, the obstructive respiratory events were more prominent during REM sleep but there was a subgroup of adolescents (25.3%) that showed NREM predominance of the obstructive respiratory events that is consistent with prior findings in pediatric studies in which NREM predominance of the obstructive respiratory events ranged between 16.7% and 30.4%.5,6 These studies showed different results regarding the characteristics of the children who had predominance of NREM obstructive events. Verginis et al.5 found that those children tend to be older, with higher arousal indices and less severe desaturations. Although Spruyt et al.6 found that this group tend to have milder desaturations, there was no effect of age or arousal indices. In our cohort, there was no effect of age, arousal indices, or oxygen desaturations.
Although in the adult population REM sleep disordered breathing was described to be more predominant in females with a sex ratio of approximately 3:1, our adolescent cohort did not show an effect of sex on the distribution of the obstructive respiratory events between REM and NREM sleep.14,19,20 However, there was an ethnicity effect on such distribution. African American adolescents had more obstructive events during REM sleep and this predominance remained significant after adjustment for BMI z-score. This is in contrast to a prior study in which African American race was described as having an association with NREM predominance of obstructive events in children aged 7.3 ± 1.2 y.6
Although obstructive events in adults with OSA tend to be worse in the supine position, this finding is less consistent in pediatric literature.7 Pereira et al.8 found that in children younger than 3 y with OSA, the respiratory disturbance index increased with the increase in time spent in supine sleep but in a later study assessing infants with the age range between 8 to 12 months, the authors found no significant difference between mean supine AHI and non-supine AHI.8,9 Another study that included children aged 1 to 10 y with OSA suggested that there were less obstructive events in the supine position.10 Cuhadaroglu et al.11 evaluated the effect of sleep position in a small cohort of non-obese children with a mean age of 6.2 y and they found that AHI was highest in lateral position in children with adenoid hypertrophy, whereas in children with adenotonsillar hypertrophy the AHI was found to be higher in the supine position due to the possible gravity effect on tonsillar tissue.11 In a subsequent study, obstructive events were found to be more predominant in the supine position, with no effect of tonsillar size on positional distribution of the obstructive events.12 Despite these different results, all of these studies targeted younger age range than our adolescent cohort. In our cohort, the positional distribution of obstructive respiratory events was similar to the adult population, with more obstructive events occurring in the supine position.
In conclusion, the obstructive respiratory events in our adolescent cohort was more prominent in REM sleep and during supine position. The REM obstructive events were more prominent in African American adolescents. There is a paucity of data on the effect of sleep state and position on the distribution of obstructive respiratory events in adolescent population. More studies are needed to further explore such effects on sleep disordered breathing in adolescents.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. All authors contributed to this manuscript.
ABBREVIATIONS
- AASM
American Association of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- EEG
electroencephalogram
- EKG
electrocardiogram
- IQR
interquartile range
- NREM
non-rapid eye movement
- OAHI
Obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- REM
rapid eye movement
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol. 1998;84:1926–36. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 4.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–6. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 5.Verginis N, Jolley D, Horne RS, Davey MJ, Nixon GM. Sleep state distribution of obstructive events in children: is obstructive sleep apnoea really a rapid eye movement sleep-related condition? J Sleep Res. 2009;18:411–4. doi: 10.1111/j.1365-2869.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 6.Spruyt K, Gozal D. REM and NREM sleep-state distribution of respiratory events in habitually snoring school-aged community children. Sleep Med. 2012;13:178–84. doi: 10.1016/j.sleep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon A, Kumar M. Influence of body position on severity of obstructive sleep apnea: a systematic review. ISRN Otolaryngol. 2013 Oct;8:670381. doi: 10.1155/2013/670381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira KD, Roebuck JC, Howell L. The effect of body position on sleep apnea in children younger than 3 years. Arch Otolaryngol Head Neck Surg. 2005;131:1014–6. doi: 10.1001/archotol.131.11.1014. [DOI] [PubMed] [Google Scholar]
- 9.Pereira KD, Rathi NK, Fatakia A, Haque SA, Castriotta RJ. Body position and obstructive sleep apnea in 8-12-month-old infants. Int J Pediatr Otorhinolaryngol. 2008;72:897–900. doi: 10.1016/j.ijporl.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes do Prado LB, Li X, Thompson R, Marcus CL. Body position and obstructive sleep apnea in children. Sleep. 2002;25:66–71. doi: 10.1093/sleep/25.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Cuhadaroglu C, Keles N, Erdamar B, et al. Body position and obstructive sleep apnea syndrome. Pediatr Pulmonol. 2003;36:335–8. doi: 10.1002/ppul.10366. [DOI] [PubMed] [Google Scholar]
- 12.Dayyat E, Maarafeya MM, Capdevila OS, Kheirandish-Gozal L, Montgomery-Downs HE, Gozal D. Nocturnal body position in sleeping children with and without obstructive sleep apnea. Pediatr Pulmonol. 2007;42:374–9. doi: 10.1002/ppul.20590. [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 14.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–64. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 15.Charbonneau M., Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–1701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- 16.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 17.Maquet P, Laureys S, Peigneux P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–6. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui F, Walters AS, Goldstein D, Lahey M, Desai H. Half of patients with obstructive sleep apnea have a higher NREM AHI than REM AHI. Sleep Med. 2006;7:281–5. doi: 10.1016/j.sleep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor C, Thornley K, Hanly P. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 20.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]