Abstract
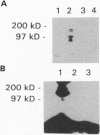
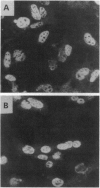
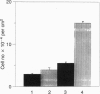
Fibroblast cell lines were established from mouse embryos homozygous for a targeted disruption of the Igf1r gene, encoding the type 1 receptor for insulin-like growth factor I (IGF-I) and from their wild-type littermates. The cells from the wild-type embryos (W cells) grow in serum-free medium supplemented with platelet-derived growth factor, epidermal growth factor, and IGF-I, whereas the cells from Igf1r(-/-) embryos (R- cells) do not, although they grow at a reduced rate in 10% fetal calf serum. The simian virus 40 (SV40) large T antigen, expressed from a transfected plasmid, can transform W cells, which form foci in monolayer cultures and colonies in soft agar (anchorage-independent growth). In contrast, the SV40 large tumor antigen, although normally expressed from the transfected template, is unable to transform R- cells, which remain contact-inhibited and fail to grow in soft agar. The transformed phenotype is restored if the R- cells carrying the SV40 large tumor antigen are stably transfected with a plasmid expressing the human IGF-I receptor. These results demonstrate that signaling via the IGF-I receptor is an indispensable component of the SV40 transformation pathway. This conclusion is further supported from the results of antisense RNA experiments with tumor cell lines showing that interference with the function of the IGF-I receptor has a profound effect on anchorage-independent growth, even under conditions that only modestly affect growth in monolayers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Baserga R. The double life of the IGF-1 receptor. Receptor. 1992 Winter;2(4):261–266. [PubMed] [Google Scholar]
- Chen S., Paucha E. Identification of a region of simian virus 40 large T antigen required for cell transformation. J Virol. 1990 Jul;64(7):3350–3357. doi: 10.1128/jvi.64.7.3350-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. J., Yee D., Rosen N. Insulinlike growth factors in human malignancy. Cancer Invest. 1991;9(4):443–454. doi: 10.3109/07357909109084643. [DOI] [PubMed] [Google Scholar]
- Deppert W., Kurth M., Graessmann M., Graessmann A., Knippschild U. Altered phosphorylation at specific sites confers a mutant phenotype to SV40 wild-type large T antigen in a flat revertant of SV40-transformed cells. Oncogene. 1991 Oct;6(10):1931–1938. [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber A., Chang C., Sell C., Ptasznik A., Cristofalo V. J., Hubbard K., Ozer H. L., Adamo M., Roberts C. T., Jr, LeRoith D. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J Biol Chem. 1993 Aug 25;268(24):17883–17888. [PubMed] [Google Scholar]
- Floros J., Jonak G., Galanti N., Baserga R. Induction of cell DNA replication in G1-specific ts mutants by microinjection of SV40 DNA. Exp Cell Res. 1981 Mar;132(1):215–223. doi: 10.1016/0014-4827(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Hollingsworth R. E., Jr, Chen P. L., Lee W. H. Integration of cell cycle control with transcriptional regulation by the retinoblastoma protein. Curr Opin Cell Biol. 1993 Apr;5(2):194–200. doi: 10.1016/0955-0674(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierstead T. D., Tevethia M. J. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J Virol. 1993 Apr;67(4):1817–1829. doi: 10.1128/jvi.67.4.1817-1829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Onwuta U. S., Lee Y. I., Li R., Botchan M. R., Robbins P. D. The retinoblastoma gene product regulates Sp1-mediated transcription. Mol Cell Biol. 1992 Jun;12(6):2455–2463. doi: 10.1128/mcb.12.6.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma L. M., Weber M. J. Constitutive phosphorylation of the receptor for insulinlike growth factor I in cells transformed by the src oncogene. Mol Cell Biol. 1990 Jul;10(7):3626–3634. doi: 10.1128/mcb.10.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. D., Rosenfeld R. G., Hintz R. L., Smith S. D. Characterization of insulin, insulin-like growth factors I and II, and growth hormone receptors on human leukemic lymphoblasts. J Clin Endocrinol Metab. 1986 Jan;62(1):28–35. doi: 10.1210/jcem-62-1-28. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Macaulay V. M. Insulin-like growth factors and cancer. Br J Cancer. 1992 Mar;65(3):311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Mayo M. W., Algate P. A., Dellow R. A., Kaleko M. Growth-promoting effects of insulin-like growth factor-1 (IGF-1) on hematopoietic cells: overexpression of introduced IGF-1 receptor abrogates interleukin-3 dependency of murine factor-dependent cells by a ligand-dependent mechanism. Blood. 1991 Aug 15;78(4):921–929. [PubMed] [Google Scholar]
- Nilson L. A., DiMaio D. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol Cell Biol. 1993 Jul;13(7):4137–4145. doi: 10.1128/mcb.13.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Porcu P., Ferber A., Pietrzkowski Z., Roberts C. T., Adamo M., LeRoith D., Baserga R. The growth-stimulatory effect of simian virus 40 T antigen requires the interaction of insulinlike growth factor 1 with its receptor. Mol Cell Biol. 1992 Nov;12(11):5069–5077. doi: 10.1128/mcb.12.11.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radna R. L., Caton Y., Jha K. K., Kaplan P., Li G., Traganos F., Ozer H. L. Growth of immortal simian virus 40 tsA-transformed human fibroblasts is temperature dependent. Mol Cell Biol. 1989 Jul;9(7):3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick-Silverman L., Pang Z., Li G., Jha K. K., Ozer H. L. Retinoblastoma protein and simian virus 40-dependent immortalization of human fibroblasts. J Virol. 1991 Jun;65(6):2845–2852. doi: 10.1128/jvi.65.6.2845-2852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979 Apr;99(1):43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- Surmacz E., Nugent P., Pietrzkowski Z., Baserga R. The role of the IGF1 receptor in the regulation of cdc2 mRNA levels in fibroblasts. Exp Cell Res. 1992 Apr;199(2):275–278. doi: 10.1016/0014-4827(92)90435-b. [DOI] [PubMed] [Google Scholar]
- Symonds H. S., McCarthy S. A., Chen J., Pipas J. M., Van Dyke T. Use of transgenic mice reveals cell-specific transformation by a simian virus 40 T-antigen amino-terminal mutant. Mol Cell Biol. 1993 Jun;13(6):3255–3265. doi: 10.1128/mcb.13.6.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco D., Fischer-Fantuzzi L., Vesco C. Limits of transforming competence of SV40 nuclear and cytoplasmic large T mutants with altered Rb binding sequences. Oncogene. 1993 Mar;8(3):549–557. [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. L., Kalderon D., Smith A. E., Tevethia M. J. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology. 1990 Sep;178(1):15–34. doi: 10.1016/0042-6822(90)90375-2. [DOI] [PubMed] [Google Scholar]
- Trojan J., Blossey B. K., Johnson T. R., Rudin S. D., Tykocinski M., Ilan J., Ilan J. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori T., Iizuka Y., Takayama Y., Nishiya S., Iwashita S., Yamazaki A., Takatori T., Tsuruo T. Insulin-like growth factor I rapidly induces tyrosine phosphorylation of a Mr 150,000 and a Mr 160,000 protein in highly metastatic mouse colon carcinoma 26 NL-17 cells. Cancer Res. 1991 Nov 1;51(21):5859–5865. [PubMed] [Google Scholar]