Fig. 2. Local and global structures of tropoelastin.
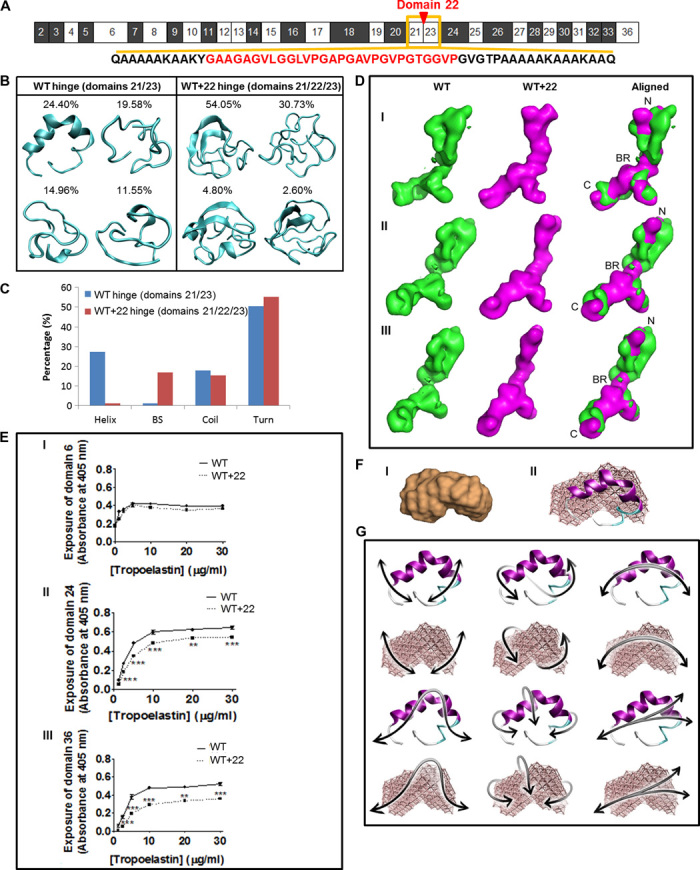
(A) Domain structure of WT. The amino acid sequences of the domain 21/23 hinge (black) and the quiescent domain 22 (red) are shown. (B) Representative structures of WT and WT+22 hinge region. Percentages indicate the significance of the cluster from which the lowest free energy representative conformation is extracted. (C) Secondary structure content [helix, β strand (BS), coil, turn] compared for molecular models of the hinge regions of WT and WT+22 constructs. (D) SAXS analysis of WT and WT+22 solution structures. Panels I, II, and III show the WT (green), WT+22 (magenta), and merged structures rotated around the long axis of the molecule. The tropoelastin N terminus (N), bridge region (BR), and C terminus (C) are indicated. The shape of the WT control is comparable to published findings (3). (E) Enzyme-linked immunosorbent assay of WT and WT+22 tropoelastin. The primary antibody used was targeted against (I) domain 6, (II) domain 24, or (III) domain 36. (F) (I) SAXS-derived structure of domains 21/23. (II) Overlay of the SAXS-based elastic network model of the domain 21/23 hinge region with the full-atomistic prediction from REMD. (G) Domain motions of the first six lowest-frequency modes of motion for the hinge region, shown for the full-atomistic model in cartoon representation (top) and the SAXS-derived elastic network model (bottom).
