Fig. 3. Dynamics of the tropoelastin molecule.
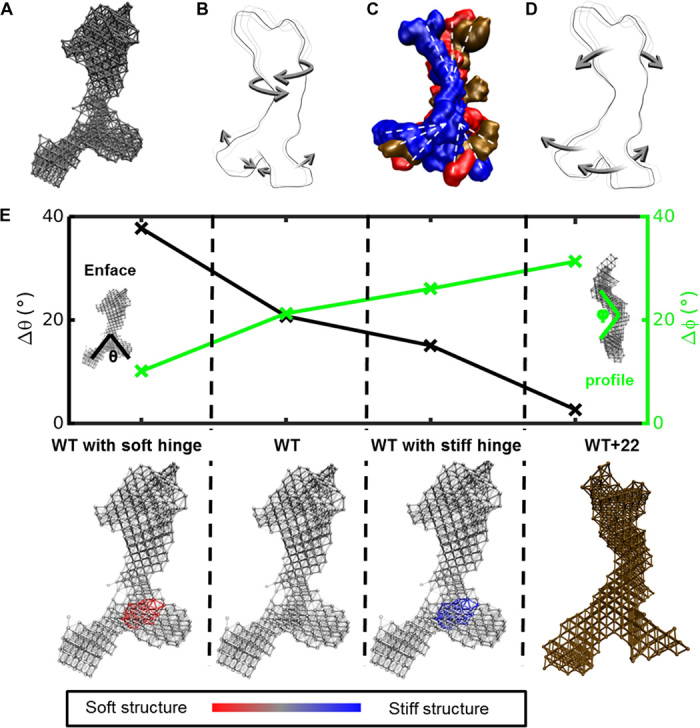
(A) Elastic network model of a SAXS-based structure of tropoelastin. (B) Tropoelastin domain motion from the linear combination of the first six lowest-frequency modes of motion, depicting the twist of the N-terminal coil region and the scissors-like flexion of the hinge and foot regions. (C) Representative solution shapes of wild-type tropoelastin (WT) showing conformations consistent with the range of molecular motions defined by the elastic network model. (D) Domain motion of WT+22 tropoelastin, characterized by the N-terminal bend along the perpendicular axis of the body and the parallel motion between the hinge region and the foot region. (E) Change in representative angles θ (between the legs of the molecule; measured enface) and φ (at the bend of the body of the molecule; measured in profile) characterizes the molecule’s dynamics for four models: WT, mutant tropoelastin (WT+22), WT with a stiffened hinge region representative of α helix–to–β sheet transition based on the full atomic-resolution models (WT with stiff hinge), and WT with a softened hinge region as an alternate control (WT with soft hinge). Corresponding model representations are presented below. For wild-type and modified hinge wild-type models, the color bar indicates relative stiffness in the structure.
