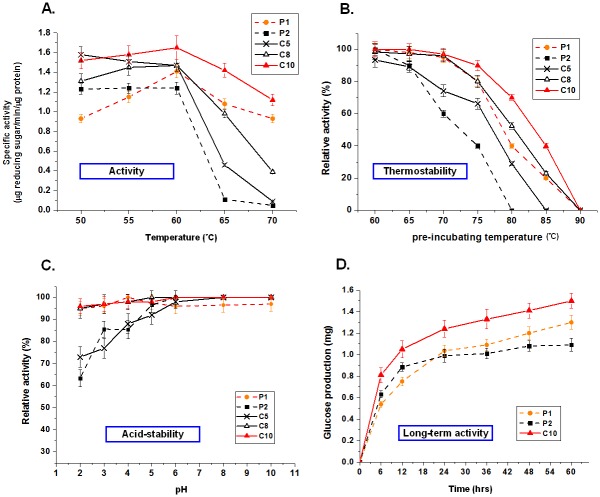
Fig 3. Characterization of GH5 chimeras.
(A) Activity profiles of GH5 parents and chimeras at high temperatures. (B) Residual activities (thermostability) of GH5 parents and chimeras after heat-treatment. One microgram of purified cellulases was pre-incubated in 50 mM sodium acetate buffer (pH 5.0) at different temperatures for 10 min. The residual enzyme activities were then determined on 1% PASC at pH 5.0 at their optimal temperature for 6 hrs. Relative activities were compared with the optimal activity of each enzyme without a pre-incubating treatment at 60°C. Some of the enzymes, e.g. C5, do not have the initial point (60°C) of 100% because their optimal activities are not at this reaction temperature and therefore have a slightly lower staring points in the analyses with 60°C pre-incubation temperature. (C) Acid stability of the parents and chosen chimeras. One microgram of purified cellulases was pre-incubated in 50 mM buffer with variable pH for 12 hrs. The residual enzyme activity was determined. Relative activities were compared with the optimal activity of each enzyme without a pre-incubating treatment at pH 5. (D) Long-term activity of the most stable chimera C10 compared with its parents in PASC hydrolysis. One microgram of purified cellulases plus 0.5 μg Novo-188 (β-glucosidase, Novozyme) was added into 1% PASC at 50°C, pH 5.0 for 6, 12, 24, 36, 48, and 60 hrs.