Abstract
Lower (versus higher) IQ scores have been shown to increase the risk of early mortality, however, the underlying mechanisms are poorly understood and previous studies underrepresent individuals with intellectual disabilities and women. This study followed one-third of all senior-year students (approximately aged 17) attending public high school in Wisconsin, USA in 1957 (n=10,317) until 2011. Men and women with mild intellectual disabilities had increased rates of mortality compared to people with the highest IQs, particularly for cardiovascular disease. Importantly, when educational attainment was held constant, people with intellectual disabilities did not have higher mortality by age 70 than people with higher IQs. Individuals with intellectual disabilities likely experience multiple disadvantages throughout life that contribute to increased risk of early mortality.
Keywords: Intellectual disability, mortality, cohort study, population-based, IQ, intelligence
Introduction
People with mild intellectual deficits (i.e., those with an IQ less than 85) comprise 16% of the general population and experience numerous disadvantages throughout adulthood. Compared to individuals with higher intellectual ability, those with lower intellectual ability experience more limitations in daily activities and greater declines in cognitive or physical functioning, are less likely to work or have less prestigious occupations and lower incomes, and have worse psychological well-being and greater rates of depression. (Bosma et al. 2007; Fujiura 2003; Seltzer et al. 2005) Moreover, parents of children with mild intellectual deficits have lower expectations for their children’s achievement, compared to parents of children with greater intellectual ability. (Taylor et al.2010; Jokela et al. 2009) Perhaps the ultimate disadvantage linked to lower intellectual ability is earlier mortality; multiple studies have demonstrated an overall inverse association between early-life IQ and later risk of mortality (Calvin et al. 2011 presents a review of previous studies), however, the specific mechanisms for this association remain unclear.
As described by Deary (2008), researchers have advanced four potential explanations for the excess risk of mortality among people with lower IQ scores compared to those with higher IQ scores. First, it has been hypothesized that people with higher IQ scores are more likely to live in healthier environments than people with lower IQ scores. Interestingly, adjusting for family socioeconomic status in childhood does little to change the association between IQ and mortality in most previous studies. (Calvin et al. 2011) In addition, individuals with higher IQ scores are more likely to receive more education and obtain higher-paying jobs and better working conditions. Several studies demonstrated that adjusting for socioeconomic status in adulthood attenuated the relationship between IQ and mortality (Deary et al. 2008; Hart et al. 2003; Batty et al. 2008; Jokela et al. 2009; Hemmingsson et al 2006), but the appropriateness of this adjustment is debated (Deary et al. 2008, Vagero 2011).
A second explanation is that individuals with lower IQ scores are less likely to practice healthy behaviors (such as having a healthy diet, not smoking, avoiding injury, and accessing health care) compared to people with higher IQ scores, and IQ may be differentially related to different causes of mortality. (Deary 2008, Gottfredson 2004) Several studies, including a Scottish cohort, a Swedish cohort of 1 000 000 men, and a cohort of US military personnel have examined the relationship between IQ and cause-specific mortality. Notably, all three studies reported that cardiovascular disease and heart disease mortality were higher among those with lower IQ versus higher IQ. (Batty et al. 2009; Batty et al. 2006, Hart et al. 2003, Batty et al. 2008) However, of these cause-specific mortality studies, only one included women, and did not present women’s mortality separately from men’s. (Hart et al. 2003) An alternate view proposes that “conscientiousness”, or “dependability” are important contributors to healthier behaviors, independent from IQ. Deary et al. (2008) Hauser and Palloni (2011) used data from the Wisconsin Longitudinal Study to argue that personality characteristics— operationalized as high school class rank—were much stronger predictors of mortality than IQ.
The third hypothesis is that lower IQ scores may reflect a previous underlying syndrome or health condition that is responsible for both low IQ and increased mortality risk. For example, individuals Down syndrome are at greatly increased risk of mortality caused by unrepaired congenital heart defects as well as Alzheimer’s disease. (Yang et al. 2002) Congenital heart defects, Alzheimer’s, and intellectual disability are all attributable to the underlying genetic condition. Finally, the fourth theory suggests that high IQ is a sign of a “particularly well-wired system”, indicating particularly good health and robustness to future injuries or disease. (Deary et al. 2008) There is currently little direct evidence supporting either of these mechanisms, apart from specific syndromes.
The extant literature leaves several key areas under-explored; in particular, few studies have explicitly estimated the excess risk of mortality among individuals with mild intellectual disabilities. Several studies, due to the nature of their cohorts (for instance, cohorts of military personnel), may tend to exclude individuals with IQ scores below a certain threshold. Additionally, women have been under-represented in studies of IQ and mortality, particularly in studies examining specific causes of mortality. Lastly, many of the largest cohorts only followed individuals through middle adulthood (ages 30-55), when relatively few deaths occurred. As Hauser and Palloni (2011) mortality attributed to low IQ among younger cohorts may only account for a tiny fraction of the total mortality that will ultimately occur.
The unique features of the Wisconsin Longitudinal Study (WLS) offer the opportunity to address several of the aforementioned gaps in the literature. The WLS is composed of a population-based sample of over 10,000 individuals followed from adolescence through age 70, and includes a substantial number of individuals with mild intellectual disabilities (IQ scores <85). The WLS has also made linkages to cause of death information obtained from death certificates, which has not been previously analyzed with regard to IQ. Furthermore, the WLS is one of a few large cohorts that includes women, and—by far—the largest female cohort followed past age 55.
In the present study, we evaluate the relationship between low IQ (measured in adolescence) and age of death by age 70 in this prospective, population-based cohort of Wisconsin high school students. A second objective is to examine whether the IQ-mortality gradient differs depending on the stated ‘causes’ of death among men and women. Finally, we evaluate the relationship between IQ, educational attainment, and risk of mortality.
Methods
The Wisconsin Longitudinal Study
The Wisconsin Longitudinal Study (WLS) is a prospective cohort study that contains a random selection of 1/3 of all high school juniors living in Wisconsin in 1956 (n=10 317). At the time, approximately 75% of Wisconsin appropriately-aged children attended high school; most participants were 16 or 17 years old in 1956 when the study began, and the majority graduated high school the following year. Although some individuals did not continue to participate in subsequent surveys, adolescent variables (such as IQ) and mortality linkages were available for the entire cohort. Additional variables, including a composite variable for family socioeconomic status, month and year of birth, and participant age at IQ testing were used in our analysis. A large majority of individuals had taken the IQ test in May, 1956. For the small number of individuals that were missing information on their age when the IQ test was administered; we assumed a test date (and entry into the study) of May, 1956.
Measure of IQ
All students in Wisconsin were administered the Henmon-Nelson Test of mental ability during their freshman year (age 14-15 years) and junior year (age 16-17 years). The Henmon-Nelson is a 30-minute test of 90 problems presented in increasing order of difficulty. Junior-year Henmon-Nelson scores were available for 9508 (92.2%) of the sample. Raw Henmon-Nelson test scores were converted to IQ scores (normed to 100) based on centile rank. For the remainder without junior-year scores, 594 (5.8% of all WLS participants) had freshman-year Henmon-Nelson scores and the final 215 (2.1% of WLS participants) had other scores obtained from the Wisconsin State Testing Service. Scores from earlier years or other tests were converted to equivalent junior-year Henmon-Nelson IQ scores. Extensive documentation about the conversion process and the psychometric properties of the IQ measures are available from the WLS Project website and previous publications. (Wisconsin Longitudinal Study Documentation 1996, 1997, and 1998)
The mean IQ for the WLS sample was 100.5 and the standard deviation was 14.9 points. IQ scores ranged from 61-145. We grouped IQ into categories spanning 1 standard deviation (<=70, 71-85, 86-100, 101-115, 116-130, >130). To facilitate comparison to previous studies, we chose to display results with the highest IQ group (>130) as the referent category, along with a continuous parameterization scaled to a 1 standard deviation decrease in IQ.
Ascertainment of timing and cause of mortality
The WLS collected mortality information from several sources. It periodically performs linkages with the National Death Index and Social Security Administration’s death index to identify participants who have died during the years in which these data are available. Mortality was also recorded as part of periodic WLS surveys (1975, 1992, 2004, 2010) when family members of deceased participants were asked about the status of participants. In the all-cause analysis, data were censored at a participant’s age at time of death or May 31, 2011, whichever came first. In total, 2,069 (20.1%) of the 10,317 WLS participants are known to have died by May 31, 2011. For 73 (3.5%) of the 2069 deaths, the exact date of the death was unknown. 5 of the 73 deaths lacked any date information and were excluded. The remaining 68 deaths were known to occur before or after certain dates. 49 of these 68 deaths occurred before 1975. For these, we assigned a date of death using the available information about when the death occurred (e.g., if a death was known to occur “before 1975”, we assigned 1974; for a death occurring “after 1963”, we assigned a 1964 date; deaths known to occur during an interval were assigned the middle year as the date of death).
Information about the causes of death was available for the years 1979-2009 via record linkages to the National Death Index. National Death Index linkages indicated 1577 WLS participant deaths occurred between 1979 to 2009 (inclusive), compared to 1605 deaths ascertained using all sources of information available to WLS. The 28 individuals who died during this time did not have cause of death information and were therefore excluded. These 28 deaths were evenly distributed across the 30 year interval, the individuals had a mean IQ of 100.5, and were 50% male.
The National Death Index provides International Classification of Disease (ICD) codes for the primary “cause” of death: ICD-9 codes for deaths between 1979 and 1998, and ICD-10 codes for deaths between 1999-2009. We grouped these codes into categories: injury, cancer (with a subgroup analysis of lung versus other cancers), and cardiovascular disease. Altogether, 1,214 (77%) of the deaths fell into one of these categories. The categorization of specific ICD codes is described in the appendix.
Educational Attainment by 1975
In 1975, when most study participants were in their late 30s, the WLS asked participants to report their highest level of educational attainment. We categorized the responses as high school, some college or vocational school, and a four-year college degree or more.
Statistical Analysis
We calculated the cumulative mortality by IQ category and sex at 5-year age intervals from age 45 through age 70. Simple relative risks with 95% confidence intervals were computed to compare mortality within each group to the mortality in the group with the highest IQ. Cochran-Armitage tests were used to assess trends across IQ group categories. We additionally examined IQ as a continuous measure by plotting the cumulative mortality through age 70 for each IQ score value in men and women. We then used Cox proportional hazard models to better account for each participant’s ages while under observation and to control for background family socioeconomic status. We performed the analysis of cause-specific mortality using the Cox regression approach, but adjusted each subject’s years under observation to reflect the 1979-2009 period when the National Death index data were available. We tested the proportional hazards assumption for the regression models using the cox.zph function in the R survival package, and found no evidence that this assumption was violated.
To evaluate whether educational attainment attenuated the risk of mortality, we stratified each IQ category by the educational attainment group and determined the cumulative incidence of mortality through age 70 within each IQ/education stratum. We performed chi-square tests to determine whether the proportion of mortality by age 70 differed by IQ category within each educational level. All analyses were performed in R (R Foundation for Statistical Computing).
Results
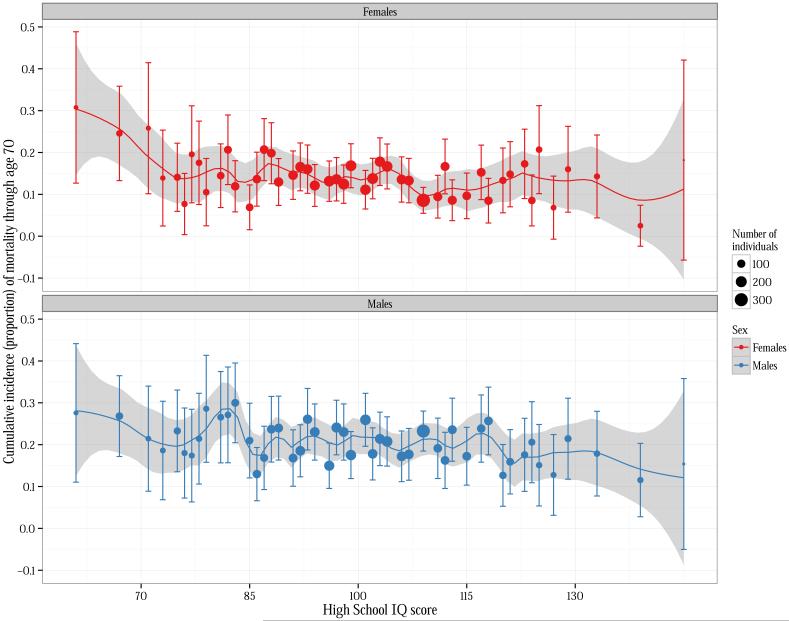
The cumulative incidence of mortality at different ages, stratified by IQ group and sex, is shown in Table 1. By age 50, and at subsequent ages, men and women in the lowest IQ group (61-70) consistently had the higher mortality than men with higher IQ scores. Men and women in the highest IQ group (131-145) tended to have the lowest cumulative mortality at all ages. At age 70, a significant trend was apparent for both men and women (Cochran Armitage p-value <0.01), suggesting a gradient in mortality across IQ categories. The proportion of men and women who died by age 70 for each individual IQ score is displayed in Figure 1.
Table 1.
Cumulative mortality of WLS participants through different ages, by IQ category and sex.
| By Age 45 | By age 50 | By age 55 | By age 60 | By age 65 | By age 70 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQ group | Total N |
% Died |
Relative Risk | % Died |
Relative Risk | % Died |
Relative Risk | % Died |
Relative Risk | % Died |
Relative Risk | % Died |
Relative Risk |
| Males | |||||||||||||
| 61-70 | 111 | 4.5 | 1.35 (0.35-5.70) | 7.2 | 1.74 (0.57-5.33) | 11.7 | 1.77 (0.73-4.27) | 16.2 | 1.78 (0.84-3.78) | 24.3 | 2.10 (1.10-4.01) | 27.0 | 1.82 (1.01-3.26) |
| 71-85 | 653 | 4.1 | 1.21 (0.47-4.19) | 6.3 | 1.52 (0.60-3.85) | 8.4 | 1.27 (0.61-2.67) | 12.4 | 1.36 (0.73-2.56) | 16.5 | 1.43 (0.92-2.49) | 23.6 | 1.59 (0.97-2.58) |
| 86-100 | 1660 | 3.9 | 1.12 (0.46-3.78) | 5.1 | 1.22 (0.50-3.02) | 6.6 | 1.00 (0.49-2.05) | 9.5 | 1.04 (0.56-1.92) | 14.3 | 1.24 (0.72-2.12) | 20.2 | 1.36 (0.85-2.19) |
| 101-115 | 1743 | 4.4 | 1.27 (0.53-4.25) | 5.3 | 1.28 (0.52-3.14) | 7.7 | 1.16 (0.57-2.37) | 11.3 | 1.24 (0.68-2.28) | 16.1 | 1.39 (0.81-2.37) | 20.5 | 1.38 (0.86-2.22) |
| 116-130 | 701 | 3.6 | 1.04 (0.40-3.63) | 5.4 | 1.31 (0.52-3.33) | 7.4 | 1.12 (0.53-2.36) | 10.8 | 1.19 (0.63-2.24) | 15.1 | 1.31 (0.75-2.28) | 19.3 | 1.29 (0.79-2.21) |
| 131-145 | 121 | 3.3 | 1.00 (Ref) | 4.1 | 1.00 (Ref) | 6.6 | 1.00 (Ref) | 9.1 | 1.00 (Ref) | 11.6 | 1.00 (Ref) | 14.9 | 1.00 (Ref) |
|
| |||||||||||||
| Cochran-Armitage p-value | 0.72 | 0.34 | 0.42 | 0.38 | 0.17 | 0.01 | |||||||
|
| |||||||||||||
| Females | |||||||||||||
| 61-70 | 83 | 2.4 | - | 7.2 | 7.23 (0.87--60.0) | 13.3 | 4.42 (1.23-15.8) | 14.5 | 2.89 (1.02-8.21) | 18.1 | 2.26 (0.96-5.33) | 26.5 | 2.65 (1.26-5.60) |
| 71-85 | 721 | 1.9 | - | 2.8 | 2.77 (0.37-20.7) | 4.2 | 1.39 (0.42-4.54) | 7.2 | 1.44 (0.58-3.61) | 9.8 | 1.23 (0.59-2.56) | 14.1 | 1.41 (0.74-2.71) |
| 86-100 | 1865 | 2.7 | - | 4.1 | 4.08 (0.57-29.3) | 5.4 | 1.79 (0.57-5.64) | 7.3 | 1.47 (0.60-3.59) | 10.5 | 1.31 (0.64-2.65) | 15.0 | 1.50 (0.80-2.81) |
| 101-115 | 1859 | 2.4 | - | 3.0 | 2.96 (0.41-21.3) | 4.6 | 1.52 (0.48-4.82) | 6.6 | 1.31 (0.54-3.21) | 9.2 | 1.15 (0.57-2.34) | 12.5 | 1.25 (0.67-2.36) |
| 116-130 | 695 | 1.9 | - | 3.0 | 3.02 (0.41-22.5) | 4.5 | 1.49 (0.45-4.86) | 6.0 | 1.21 (0.48-3.05 | 9.2 | 1.15 (0.55-2.40) | 13.4 | 1.34 (0.70-2.57) |
| 131-145 | 100 | 0.0 | Ref | 1.0 | 1.00 (Ref) | 3.0 | 1.00 (Ref) | 5.0 | 1.00 (Ref) | 8.0 | 1.00 (Ref) | 10.0 | 1.00 (Ref) |
|
| |||||||||||||
| Cochran-Armitage p-value | 0.39 | 0.10 | 0.10 | 0.03 | 0.06 | 0.01 | |||||||
Figure 1.
Proportion of women and men who died by age 70, by IQ score. A loess smoothing line (and locally-weighted 95% confidence band) is displayed behind the IQ scores. Vertical gray lines represent the boundaries for the IQ categories used in this analysis.
The Cox proportional hazards analysis incorporated the full time under observation for each individual, including known deaths occurring beyond age 70. The hazard ratios for men (Table 2, left side) are comparable to the risk ratios in the cumulative mortality analysis by age 70 (Table 1). Men in the 61-70 and 71-85 IQ groups had significantly elevated mortality (HRs of 1.92 and 1.74, respectively), when compared to men in the highest IQ group. The women (Table 2, right side) also had greater mortality in the lowest IQ category (adjusted hazard ratio =2.01; 95% CI 1.08-3.76) compared to the highest IQ category, but there was little apparent variability across the rest of the IQ range. There were 6 deaths in the highest female IQ category that occurred shortly after age 70 (after the oldest age displayed in Table 1) which lead to lower relative hazards when comparing this group to the lower IQ categories. Adjusting for parental and family socioeconomic status had little effect on the association between IQ and mortality in men or women. When we treated IQ as a continuous variable, the hazard ratios for a standard deviation decrease (14.9 IQ points) were similar among men (adjusted HR=1.08, 95% CI 1.02-1.14) and women (adjusted HR=1.10, 95% CI 1.03-1.18).
Table 2.
Unadjusted and adjusted hazard ratios for mortality for men and women, by IQ group. IQ was analyzed as both a categorical and continuous variable.
| Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|
| IQ category | Unadjusted Hazard Ratio |
95% CI | Hazard Ratio Adjusted for family SES |
95% CI | Unadjusted Hazard Ratio |
95% CI | Hazard Ratio Adjusted for family SES |
95% CI |
| 61-70 | 1.92 | (1.10-3.34) | 1.88 | (1.07-3.29) | 2.13 | (1.14-3.95) | 2.01 | (1.08-3.76) |
| 71-85 | 1.74 | (1.09-2.75) | 1.70 | (1.07-2.71) | 1.03 | (0.61-1.73) | 0.98 | (0.58-1.67) |
| 86-100 | 1.47 | (0.94-2.30) | 1.45 | (0.92-2.27) | 1.11 | (0.67-1.84) | 1.07 | (0.65-1.77) |
| 101-115 | 1.46 | (0.93-2.28) | 1.44 | (0.92-2.26) | 0.95 | (0.57-1.57) | 0.92 | (0.56-1.53) |
| 116-130 | 1.34 | (0.84-2.14) | 1.34 | (0.84-2.13) | 0.95 | (0.56-1.60) | 0.93 | (0.56-1.59) |
| 131-145 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| Continuous IQ | ||||||||
| 1 SD Decrease (14.9 IQ pts) | 1.09 | (1.03-1.15) | 1.08 | (1.02-1.14) | 1.11 | (1.04-1.19) | 1.10 | (1.03-1.18) |
Cause-specific mortality using National Death Index information
Among the 1576 deaths identified by the National Death Index between the years 1979-2009, we observed an inverse association between IQ and all-cause mortality in both men and women (Table 3) that was consistent with the analysis using all available data sources and years (Table 2). Among men, a standard deviation decrease in IQ was associated with an adjusted hazard of 1.28 (95% CI: 1.00-1.65) for deaths attributable to injury. Injuries only accounted for 7.2% of male deaths. Injuries accounted for only 4.3% of female deaths and no significant trend across IQ categories was apparent for women. Suicides and homicides were included in the injury category, as there were only 5 people (4 men, 1 woman) with an ICD code for suicide and 2 (both women) for homicide. None of these individuals were in the highest, lowest, or 2nd lowest IQ categories and removing these individuals had little influence on the results for this category. Overall, there was little difference in IQ group by deaths attributable to cancer, however, the continuous (linear) IQ hazard ratio for lung cancer among women just missed statistical significance (adjusted HR = 1.25, 95% CI: 0.99-1.57).
Table 3.
Association between IQ and cause-specific mortality in men and women, among individuals with available death certificate information (years of death 1979-2009). All hazard ratios are adjusted for family socioeconomic status.
| All cause death between 1979-2009 |
Injury |
Lung & Pharynx Cancer only |
Cancers Other than Lung and Pharynx |
All Cancer |
Cardiovascular Disease |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQ category | N | N. Deaths |
Hazard Ratio |
95% CI | N. Deaths |
Hazard Ratio |
95% CI | N. Deaths |
Hazard Ratio |
95% CI | N. Deaths |
Hazard Ratio |
95% CI | N. Deaths |
Hazard Ratio |
95% CI | N. Deaths |
Hazard Ratio |
95% CI |
| Males | |||||||||||||||||||
|
| |||||||||||||||||||
| 61-70 | 105 | 25 | 1.93 | (1.01-3.68) | 1 | - | - | 2 | 1.05 | (0.15-7.55) | 7 | 0.96 | (0.35-2.69) | 9 | 0.98 | (0.39-2.43) | 9 | 3.69 | (0.99-13.8) |
| 71-85 | 632 | 140 | 1.75 | (1.02-3.01) | 12 | - | - | 16 | 1.35 | (0.30-5.98) | 26 | 0.58 | (0.26-1.30) | 42 | 0.74 | (0.36-1.48) | 54 | 3.58 | (1.11-11.6) |
| 86-100 | 1607 | 298 | 1.45 | (0.86-2.45) | 26 | - | - | 34 | 1.13 | (0.27-4.78) | 79 | 0.69 | (0.33-1.44) | 113 | 0.78 | (0.40-1.50) | 95 | 2.43 | (0.76-7.70) |
| 101-115 | 1684 | 311 | 1.48 | (0.88-2.49) | 19 | - | - | 36 | 1.20 | (0.29-5.01) | 91 | 0.78 | (0.38-1.63) | 127 | 0.87 | (0.45-1.66) | 98 | 2.41 | (0.76-7.62) |
| 116-130 | 682 | 119 | 1.40 | (0.82-2.40) | 7 | - | - | 13 | 1.12 | (0.25-4.95) | 35 | 0.76 | (0.35-1.65) | 48 | 0.83 | (0.42-1.65) | 35 | 2.09 | (0.64-6.78) |
| 131-145 | 117 | 15 | 1.00 | ref | 0 | - | ref | 2 | 1.00 | ref | 8 | 1.00 | ref | 10 | 1.00 | ref | 3 | 1.00 | ref |
| Continuous IQ | |||||||||||||||||||
| 1 SD Decrease | 4827 | 908 | 1.09 | (1.02-1.16) | 65 | 1.28 | (1.00-1.65) | 103 | 1.04 | (0.85-1.26) | 246 | 0.94 | (0.84-1.08) | 349 | 0.97 | (0.87-1.08) | 294 | 1.18 | (1.05-1.33) |
|
| |||||||||||||||||||
| Females | |||||||||||||||||||
|
| |||||||||||||||||||
| 61-70 | 81 | 21 | 2.31 | (1.11-4.83) | 1 | 0.88 | (0.08-10.1) | 1 | 0.80 | (0.07-8.93) | 6 | 3.52 | (0.71-17.6) | 7 | 2.22 | (0.65-7.65) | 8 | 4.58 | (0.96-21.8) |
| 71-85 | 710 | 96 | 1.15 | (0.61-2.15) | 3 | 0.27 | (0.04-1.71) | 18 | 1.51 | (0.34-6.64) | 32 | 2.00 | (0.48-8.40) | 50 | 1.69 | (0.60-4.70) | 21 | 1.31 | (0.30-5.63) |
| 86-100 | 1828 | 253 | 1.20 | (0.65-2.20) | 10 | 0.34 | (0.72-1.61) | 32 | 1.02 | (0.24-4.29) | 101 | 2.52 | (0.62-10.3) | 133 | 1.77 | (0.65-4.80) | 42 | 1.02 | (0.25-4.27) |
| 101-115 | 1820 | 203 | 0.98 | (0.53-1.80) | 8 | 0.25 | (0.05-1.19) | 22 | 0.66 | (0.15-2.81) | 84 | 2.16 | (0.53-8.80) | 106 | 1.42 | (0.52-3.86) | 40 | 1.03 | (0.25-4.29) |
| 116-130 | 685 | 84 | 1.11 | (0.59-2.08) | 5 | 0.39 | (0.07-2.00) | 11 | 0.82 | (0.18-3.68) | 32 | 2.31 | (0.55-9.63) | 43 | 1.57 | (0.56-4.36) | 21 | 1.52 | (0.36-6.47) |
| 131-145 | 100 | 11 | 1.00 | ref | 2 | 1.00 | ref | 2 | 1.00 | ref | 2 | 1.00 | ref | 4 | 1.00 | ref | 2 | 1.00 | ref |
| Continuous IQ | |||||||||||||||||||
| 1 SD increase | 5224 | 668 | 1.12 | (1.03-1.21) | 29 | 1.03 | (0.69-1.52) | 86 | 1.25 | (0.99-1.57) | 257 | 1.05 | (0.92-1.20) | 343 | 1.10 | (0.98-1.23) | 134 | 1.15 | (0.96-1.39) |
Mortality from cardiovascular disease was associated with lower IQ among men; a standard deviation decrease in IQ was associated with an adjusted hazard ratio of 1.18 (95% CI: 1.05-1.33). Men with IQ scores in the 71-85 range were more likely to die from cardiovascular disease than men in the highest IQ category (adjusted HR =3.58, 95% CI: 1.11-11.6). There was a similarly elevated—but not statistically significant—risk of cardiovascular disease for men in the lowest IQ category (adjusted HR = 3.69, 95% CI:0.99-13.8). Eight of the 21 female deaths in the lowest IQ group were attributed to cardiovascular disease, leading to an adjusted hazard ratio of 4.58 (95% CI: 0.96-21.8). The continuous IQ hazard ratio for cardiovascular disease deaths among women was also elevated, but narrowly missed statistical significance (adjusted HR 1.15, 95% CI: 0.96-1.39). The remaining deaths (of 201 men and 162 women) had stated causes of death that did not fit into these categories, and collectively these deaths were not significantly associated with IQ among men or women. (Data not shown.)
Educational attainment as a potential mediator
In 1975, participating WLS graduates (at this time most were in their late 30s) reported their educational attainment. The vast majority of men and women with IQ scores <=85 did not receive any education beyond high school (89%), and individuals with higher IQ scores were increasingly likely to attend college. (Table 4) Among participants who reported only a high school education, mortality risk by age 70 did not differ significantly by IQ group (chi-square p-values = 0.10 among men and 0.24 among women). When compared to individuals with the same educational attainment, people with mild intellectual disabilities were not more likely to die by age 70 than people with higher IQ scores.
Table 4.
Risk of mortality by age 70, stratified by sex, IQ group, and educational attainment reported in 1975. Includes only the participants that responded to the 1975 questionnaire (4,567 men and 5,043 women).
|
High School
|
Some College
|
College Degree or
More |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IQ Category |
Total N
participating in 1975 |
N | % of IQ Group |
% died by age 70 |
N | % of IQ Group |
% died by age 70 |
N | % of IQ Group |
% died by age 70 |
| Men | ||||||||||
| 61-70 | 97 | 93 | 95.9 | 19.4 | 4 | 4.1 | 25.0 | 0 | 0.0 | a |
| 71-85 | 562 | 479 | 85.2 | 19.0 | 51 | 9.1 | 19.6 | 32 | 5.7 | 9.4 |
| 86-100 | 1531 | 1089 | 71.1 | 18.0 | 207 | 13.5 | 11.6 | 235 | 15.3 | 19.6 |
| 101-115 | 1604 | 759 | 47.3 | 22.3 | 274 | 17.1 | 18.6 | 571 | 35.6 | 11.6 |
| 116-130 | 659 | 143 | 21.7 | 22.7 | 113 | 17.1 | 16.8 | 403 | 61.2 | 14.4 |
| 131-145 | 114 | 6 | 5.3 | 50.0 | 12 | 10.5 | 16.7 | 96 | 84.2 | 9.4 |
|
| ||||||||||
|
chi-square p-
value |
0.10 | 0.40 | 0.02* | |||||||
|
| ||||||||||
| Women | ||||||||||
| 61-70 | 73 | 69 | 94.5 | 21.7 | 2 | 2.7 | 50.0 | 2 | 2.7 | 50.0 |
| 71-85 | 658 | 590 | 89.7 | 12.9 | 49 | 7.4 | 12.2 | 19 | 2.9 | 10.5 |
| 86-100 | 1764 | 1435 | 81.3 | 14.6 | 197 | 11.2 | 13.7 | 132 | 7.5 | 8.3 |
| 101-115 | 1778 | 1115 | 62.7 | 12.7 | 274 | 15.4 | 10.9 | 389 | 21.9 | 8.2 |
| 116-130 | 671 | 282 | 42.0 | 14.1 | 117 | 17.4 | 17.1 | 272 | 40.5 | 8.5 |
| 131-145 | 99 | 26 | 26.3 | 7.7 | 12 | 12.1 | 16.7 | 61 | 61.6 | 9.8 |
|
| ||||||||||
|
chi-square p-
value |
0.24 | 0.38 | 0.46 | |||||||
Note:
Because there were no men with IQ scores 61-70 who graduated college, the chi-square statistic includes the other 5 IQ categories.
Discussion
This study provides population-based information about the risk of mortality among men and women with mild intellectual disability up to late adulthood. In terms of the four hypotheses put forth by “cognitive epidemiologists” (Deary & Batty 2007), the results of the present study provide at least some support to two. First, the increased risk of mortality for men and women with lower IQ scores was largely attenuated when compared to individuals with higher IQ scores but similar levels of education. If higher adult socioeconomic status actually reduces the risk of earlier mortality, very few of the participants with mild intellectual disability would have the opportunity to benefit by continuing education beyond high school. Second, the different gradients among specific causes of mortality could suggest health behaviors and the environment play important roles. These health behaviors could be interpreted as indirect evidence of good decision making and health literacy as suggested by Gottfredson and Deary (2004), but may also reflect the environment and limited opportunities available to an individual.
In an earlier analysis using the WLS, Hauser and Palloni (2011) observed that controlling for high school class rank fully attenuated the association between IQ and risk of mortality. They concluded that the stronger association between class rank (based on the grades a student earned) and mortality suggests that personality characteristics—in addition to IQ—better predict mortality, because there was a great deal of variability in class ranks among individuals with the same IQ score. Even for individuals with IQ <=85, the correlation coefficient between class rank and IQ is only 0.29 (data not shown); however, the variability in class rank was compressed--nearly all people with IQ <=85 were ranked in the bottom half of their high school class and very few (3.8%, Table 4) went on to graduate from a 4-year college. While having a high IQ may not be sufficient (on its own) for predicting class rank and educational achievement, some baseline level of intellectual ability seems necessary to receive further opportunities, as indicated by the educational achievement of the low-IQ group.
Similar to previous studies, our findings show lower adolescent IQ is consistently associated with higher all-cause mortality. We observed relatively higher mortality among women with IQ scores below 70 and men with IQ scores below 85, compared to those with IQ scores above 130. When IQ scores were parameterized as continuous measures, there was an overall association between mortality and decreasing IQ for both men and women, but the association is not necessarily monotonic across the IQ range. In women especially, the hazard ratios for mortality hovered around 1.00 for all IQ groups >70, and was only elevated for those with IQ 61-70. (Table 2) Among men, there was little difference between the three IQ largest groups (covering scores between 86-130) in any of the analyses.
Previous studies tended to report that IQ had a larger effect on early mortality, compared to the current study. A meta-analysis reported a hazard ratio of 1.24 for a standard deviation decrease in IQ (Calvin et al. 2011), compared to our findings for men (adjusted HR=1.08) and women (adjusted HR=1.10). One explanation for this difference could be the age to which the cohorts are followed; most of the surviving WLS cohort members were in their early 70s, whereas the large Swedish and British cohorts that were only followed until their late 30s or 40s and deaths were far less frequent. Other possible dissimilarities include the composition of the cohorts and the difference between growing up in Wisconsin in the 1950s versus Sweden or the UK.
This study was one of few population-based cohort studies with information about specific causes of death for both men and women. The proportion of all deaths due to injury and suicide is much smaller in the WLS cohort than in the Swedish studies examining these outcomes. (Batty et al. 2009) This may be attributable to the higher overall rate of mortality from the older WLS cohort, as cardiovascular and cancer mortality become much more common with increasing age. Although low IQ was a significant risk factor for death due to injury among men, only 7% of the deaths (with known causes) were attributed to injury and would explain relatively little of the inequality of mortality between men with low versus higher IQ scores.
The increased risk of cardiovascular disease among men with lower IQ is consistent with findings in other studies. (Batty 2008; Hart et al 2003). Cardiovascular researchers generally believe that a high proportion of the cardiovascular disease that occurs in the United States is preventable. (Kuller 2013) Additionally, there are critical gaps in clinical prevention (fewer than half of the people at increased risk are prescribed aspirin as a preventive measure), that many of the recommended prevention measures are considered expensive (estimated at $1,700 per person per year), and—other than smoking cessation—CVD prevention strategies not necessarily cost-effective. (Kahn et al. 2008; Tomaselli et al. 2011) While individuals with learning or intellectual disabilities may have more difficulty adhering to prescribed prevention strategies as suggested by Gottfredson (2004), the American Heart Association cites lack of access to recommended services as the largest cause of preventable heart attacks. (Tomaselli et al. 2011) Individuals with lower IQ may have less access to these services than individuals with higher IQ, as a consequence of having lower incomes.
This study has a number of important strengths, including its population-based design and duration of follow-up into late adulthood. Although not all people attended high school in Wisconsin in the 1950s (approximately 75% graduated high school at this time), this sample includes a sizeable number of men and women who are likely to have mild intellectual or learning disabilities (with IQ scores <85), ensuring a broad and generalizable range of intellectual abilities. An additional strength is the availability of cause-specific mortality information covering deaths over a 30 year range (approximately ages 39 – 69) for both men and women.
Despite its strengths, this study has several noteworthy weaknesses. Because mortality is lower in women than men at all ages throughout adulthood, we had less statistical power to examine specific causes of mortality among women than among men. The association between lower IQ and mortality caused by lung cancer and cardiovascular disease mortality did not meet the threshold for statistical significance among women, but may in the future as more events are observed. There were also some losses to follow-up as the WLS continued to collect information from these participants over the decades; while the monitoring of mortality did not require continued involvement from the participants, the collection of other information (such as educational attainment) was limited to the living and participating respondents. An additional limitation is the measurement of the categorical ‘cause’ of death from death certificate ICD codes; the primary cause of death was algorithmically determined from the listed codes, and is an imperfect measure of the most relevant (and proximal) cause of death.
In summary, this study investigated the association between low IQ and specific causes of death in men and women in a US population-based cohort. We consistently observed higher all-cause mortality among men and women with lower IQ, but the associations we observed were of smaller magnitude than many previous studies. The magnitude of the association between low IQ and cardiovascular disease mortality, however, was comparable to that observed in a previous study. When men and women with mild intellectual disabilities were compared to other with the same level of education, the risk of mortality was similar across IQ groups. Further studies in other cohorts are needed to better understand how experiences later in life are related to specific causes of mortality in men and women with mild intellectual disabilities.
Supplementary Material
Acknowledgments
This study is supported by grants from the National Institute on Aging, Project 3 of P01 AG021079 (to Marsha R. Mailick, PI) and the National Institute of Child Health and Human Development (P30 HD03352 and T32 HD07489 both to Marsha R Mailick, PI)
References
- Batty GD, et al. IQ in Early Adulthood and Mortality By Middle Age. Epidemiology. 2009;20:100–109. doi: 10.1097/EDE.0b013e31818ba076. [DOI] [PubMed] [Google Scholar]
- Batty GD, et al. Does IQ predict total and cardiovascular disease mortality as strongly as other risk factors? Comparison of effect estimates using the Vietnam Experience Study. Heart. 2008;94:1541–1544. doi: 10.1136/hrt.2008.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, et al. IQ in late adolescence/early adulthood, risk factors in middle age and later all-cause mortality in men: the Vietnam Experience Study. Journal of Epidemiology & Community Health. 2008;62(6):522–531. doi: 10.1136/jech.2007.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H, et al. To what extent does IQ “explain” socio-economic variations in function? BMC Public Health. 2007;7(1):179. doi: 10.1186/1471-2458-7-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin CM, et al. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. International Journal of Epidemiology. 2010;40(3):626–644. doi: 10.1093/ije/dyq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I. Why do intelligent people live longer? Nature. 2008;456(7219):175–176. doi: 10.1038/456175a. [DOI] [PubMed] [Google Scholar]
- Deary IJ, et al. More Intelligent, More Dependable Children Live Longer: A 55-Year Longitudinal Study of a Representative Sample of the Scottish Nation. Psychological Science. 2008;19(9):874–880. doi: 10.1111/j.1467-9280.2008.02171.x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Batty GD. Cognitive epidemiology. Journal of Epidemiology & Community Health. 2007;61:378–384. doi: 10.1136/jech.2005.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiura GT. Continuum of intellectual disability: demographic evidence for the “forgotten generation”. Mental Retardation. 2003;41:420–429. doi: 10.1352/0047-6765(2003)41<420:COIDDE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: Is It the Epidemiologists’ Elusive “Fundamental Cause” of Social Class Inequalities in Health? Journal of Personality and Social Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS, Deary IJ. Intelligence Predicts Health and Longevity, but Why?
- Hart CL. Childhood IQ, Social Class, Deprivation, and Their Relationships with Mortality and Morbidity Risk in Later Life: Prospective Observational Study Linking the Scottish Mental Survey 1932 and the Midspan Studies. Psychosomatic Medicine. 2003;65(5):877–883. doi: 10.1097/01.psy.0000088584.82822.86. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Palloni A. Adolescent IQ and Survival in the Wisconsin Longitudinal Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B:i91–i101. doi: 10.1093/geronb/gbr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsson T. The association between cognitive ability measured at ages 18-20 and mortality during 30 years of follow-up--a prospective observational study among Swedish males born 1949-51. International Journal of Epidemiology. 2006;35(3):665–670. doi: 10.1093/ije/dyi321. [DOI] [PubMed] [Google Scholar]
- Jokela M, et al. Low Childhood IQ and Early Adult Mortality: The Role of Explanatory Factors in the 1958 British Birth Cohort. PEDIATRICS. 2009;124:e380–e388. doi: 10.1542/peds.2009-0334. [DOI] [PubMed] [Google Scholar]
- Kahn R, et al. The Impact of Prevention on Reducing the Burden of Cardiovascular Disease. Circulation. 2008;118(5):576–585. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- Kuller LH. Point: Is There a Future for Innovative Epidemiology? American Journal of Epidemiology. 2013;177(4):279–280. doi: 10.1093/aje/kws414. [DOI] [PubMed] [Google Scholar]
- Lager A, Bremberg S, Vagero D. The association of early IQ and education with mortality: 65 year longitudinal study in Malmo, Sweden. BMJ. 2009;339:b5282–b5282. doi: 10.1136/bmj.b5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, et al. Life course impacts of mild intellectual deficits. American Journal on Mental Retardation. 2005;110(6):451–468. doi: 10.1352/0895-8017(2005)110[451:LCIOMI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Taylor JL, et al. Parenting With Mild Intellectual Deficits: Parental Expectations and the Educational Attainment of Their Children. American Journal on Intellectual and Developmental Disabilities. 2010;115(4):340–354. doi: 10.1352/1944-7558-115.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli GF, et al. The American Heart Association and the Million Hearts Initiative: A Presidential Advisory From the American Heart Association. Circulation. 2011;124(16):1795–1799. doi: 10.1161/CIR.0b013e3182327084. [DOI] [PubMed] [Google Scholar]
- Vagero D. Commentary: Intelligence in youth and all-cause mortality: some problems in a recent meta-analysis. International Journal of Epidemiology. 2011;40(3):644–646. doi: 10.1093/ije/dyr079. [DOI] [PubMed] [Google Scholar]
- Wisconsin Longitudinal Study Using Data on IQ and High School Rank from the WLS. 1996 Available: http://www.ssc.wisc.edu/wlsresearch/documentation/appendices/G/memo121.asc (Accessed May 8, 2013)
- Wisconsin Longitudinal Study Respondent IQ data-updates and recommendations. 1997 Available: http://www.ssc.wisc.edu/wlsresearch/documentation/appendices/G/memo124.asc and http://www.ssc.wisc.edu/wlsresearch/documentation/appendices/G/memo124.pdf. (Accessed May 8, 2013)
- Wisconsin Longitudinal Study Construction of “new” IQ variables. 1998 Available: http://www.ssc.wisc.edu/wlsresearch/documentation/appendices/G/cor652.asc. (Accessed May 08, 2013)
- Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. The Lancet. 2002;359(9311):1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.