Table 1.
Enantioselective Synthesis of Substituted Hydroxy-Oxindole Scaffolds
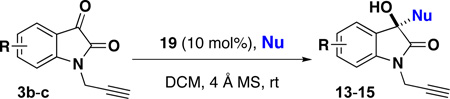 | ||||||
|---|---|---|---|---|---|---|
| entry | R | isatin | nucleophile | product | yielda | %eeb |
| 1c | 5-F | 3b | 11a | 13b | 97 | 98 |
| 2d | 5-F | 3b | 11b | 14b | 79 | 87 |
| 3 | 5-F | 3b | 11c | 15b | 97 | 96 |
| 4c | 4-Cl | 3c | 11a | 13c | 78 | 86 |
| 5d | 4-Cl | 3c | 11b | 14c | 90 | 94 |
| 6 | 4-Cl | 3c | 11c | 15c | 97 | 99 |
Isolated yields.
Determined using HPLC analysis with chiral stationary phase.
Reaction performed at −20 °C.
Reaction was performed using 3.0 equiv of TMSCl and 0.1 equiv of NaSbF6 in MeCN.
