Table 3.
Regio- and Stereoselective Spirocyclization to Afford 2- and 3-spirooxazoline Scaffolds
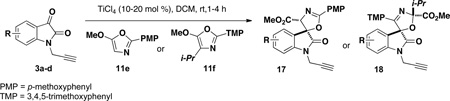 | ||||||
|---|---|---|---|---|---|---|
| entry | R | isatin | oxazole | product | yield (%)a | drb |
| 1 | 5-F | 3b | 11e | 17b | 55c | 90:10 |
| 2 | 4-Cl | 3c | 11e | 17c | 74 | 99:1 |
| 3 | H | 3a | 11f | 18a | 77c | 90:10 |
| 4 | 5-F | 3b | 11f | 18b | 87 | 93:7 |
| 5 | 4-Cl | 3c | 11f | 18c | 51c | 95:5 |
| 6 | 5-Br | 3d | 11f | 18d | 95 | 99:1 |
Isolated yield of major diastereomer.
Determined by analysis of 1H NMR spectroscopy of crude reaction mixture.
Yields sacrificed for purity due to the presence of by-products which proved to be difficult to separate by column chromatography (conversion ≥80% by TLC).
