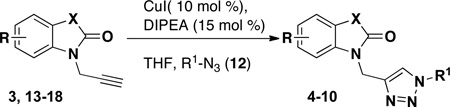
Table 4.
Synthesis of Triazole-functionalized Isatins, Oxindoles, and Spirooxindoles
 | |||||
|---|---|---|---|---|---|
| entry | R | isatin/oxindole | azide (R1) | producta | yield (%)b |
| 1 | 5-F | 13b | 12a | 4ba | 78 |
| 2 | 5-F | 13b | 12b | 4bb | 80 |
| 3 | 5-F | 13b | 12c | 4bc | 99 |
| 4 | 5-F | 14b | 12a | 5ba | 64 |
| 5 | 5-F | 14b | 12b | 5bb | 71 |
| 6 | 5-F | 15b | 12a | 6ba | 56 |
| 7 | 5-F | 15b | 12b | 6bb | 99 |
| 8 | 4-Cl | 13c | 12a | 4ca | 40 |
| 9 | 4-Cl | 13c | 12b | 4cb | 70 |
| 10 | 4-Cl | 14c | 12a | 5ca | 94 |
| 11 | 4-Cl | 14c | 12b | 5cb | 58 |
| 12 | 4-Cl | 15c | 12a | 6ca | 91 |
| 13 | 4-Cl | 15c | 12b | 6cb | 94 |
| 14 | 4-Cl | 15c | 12c | 6cc | 58 |
| 15 | H | 16a | 12c | 7ac | 95 |
| 16 | H | 16a | 12f | 7af | 95 |
| 17 | 5-F | 16b | 12b | 7bb | 62 |
| 18 | 5-F | 16b | 12c | 7bc | 97 |
| 19 | 7-F | 16f | 12a | 7fa | 70 |
| 20 | H | 3a | 12d | 8ad | 69 |
| 21 | H | 3a | 12a | 8aa | 99 |
| 22 | H | 3a | 12e | 8ae | 95 |
| 23 | 4-Cl | 3c | 12a | 8ca | 55 |
| 24 | 4-Cl | 3c | 12f | 8cf | 99 |
| 25 | 4-Cl | 3c | 12c | 8cc | 97 |
| 26 | 5-Br | 3d | 12a | 8da | 74 |
| 27 | 5-OMe | 3e | 12a | 8ea | 99 |
| 28 | 5-F | 17b | 12b | 9bb | 74 |
| 29 | 4-Cl | 17c | 12a | 9ca | 88 |
| 30 | 4-Cl | 17c | 12b | 9cb | 94 |
| 31 | 4-Cl | 17c | 12c | 9cc | 88 |
| 32 | 4-Cl | 17c | 12f | 9cf | 87 |
| 33 | 4-Cl | 17c | 12d | 9cd | 99 |
| 34 | H | 18a | 12a | 10aa | 92 |
| 35 | H | 18a | 12b | 10ab | 76 |
| 36 | H | 18a | 12f | 10af | 70 |
| 37 | H | 18a | 12d | 10ad | 90 |
| 38 | 5-F | 18b | 12a | 10ba | 80 |
| 39 | 5-F | 18b | 12b | 10bb | 74 |
| 40 | 5-F | 18b | 12c | 10bc | 70 |
| 41 | 5-F | 18b | 12e | 10be | 94 |
| 42 | 5-F | 18b | 12d | 10bd | 74 |
| 43 | 4-Cl | 18c | 12b | 10cb | 66 |
| 44 | 4-Cl | 18c | 12d | 10cd | 93 |
| 45 | 5-Br | 18d | 12a | 10da | 99 |
All reactions performed with CuI (10 mol %) and DIPEA (15 mol %) in THF from 12–24 h.
Isolated yield.
