Table 5.
Synthesis of Triazole-functionalized Oxindoles and Isatins from Aryl-iodides
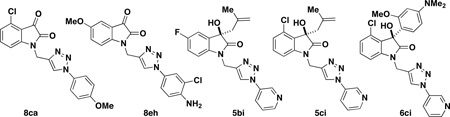 | |||||
|---|---|---|---|---|---|
| entry | R1 | isatin/oxindole | Ar-N3a | product | yield (%)b |
| 1c | 4-Cl | 3c | 12g | 8ca | 23 |
| 2c | 5-OMe | 3e | 12h | 8eh | 35 |
| 3d | 5-F | 14b | 12i | 5bi | 28 |
| 4d | 4-Cl | 14c | 12i | 5ci | 40 |
| 5d | 4-Cl | 15c | 12i | 6ci | 19 |
Azide generated in situ from the corresponding aryl-iodide.
Isolated yield.
Reactions performed using CuI (10 mol %), and DMEDA (15 mol %) according to Scheme 3, eq 3.
Reaction performed with 1 equiv of CuSO4•5H2O, and 1.1 equiv. of sodium ascorbate.
