Fig. 1.
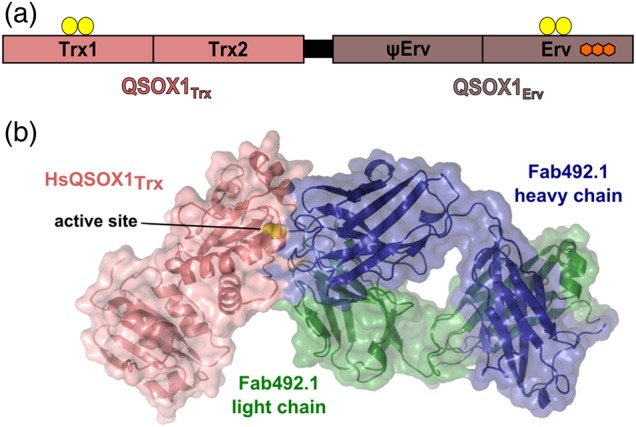
QSOX1 domain organization and inhibition by MAb492.1. (a) The two modules of mammalian QSOX1 enzymes are linked by a flexible linker (black). The amino-terminal module, QSOX1Trx, is composed of two Trx-fold domains, the first of which has a redox-active CXXC motif (yellow balls). A second redox-active CXXC motif is located in the carboxy-terminal Erv domain. This domain binds an FAD cofactor (fused hexagons). The domain labeled ψErv is a degenerate Erv domain lacking active-site cysteines and an FAD cofactor. (b) Surface presentation of a complex of a Fab fragment from MAb492.1 (Fab492.1) and HsQSOX1Trx (PDB ID: 4IJ3) showing that Fab492.1 inhibits HsQSOX1 by burying the Trx CXXC active site (yellow spheres).
