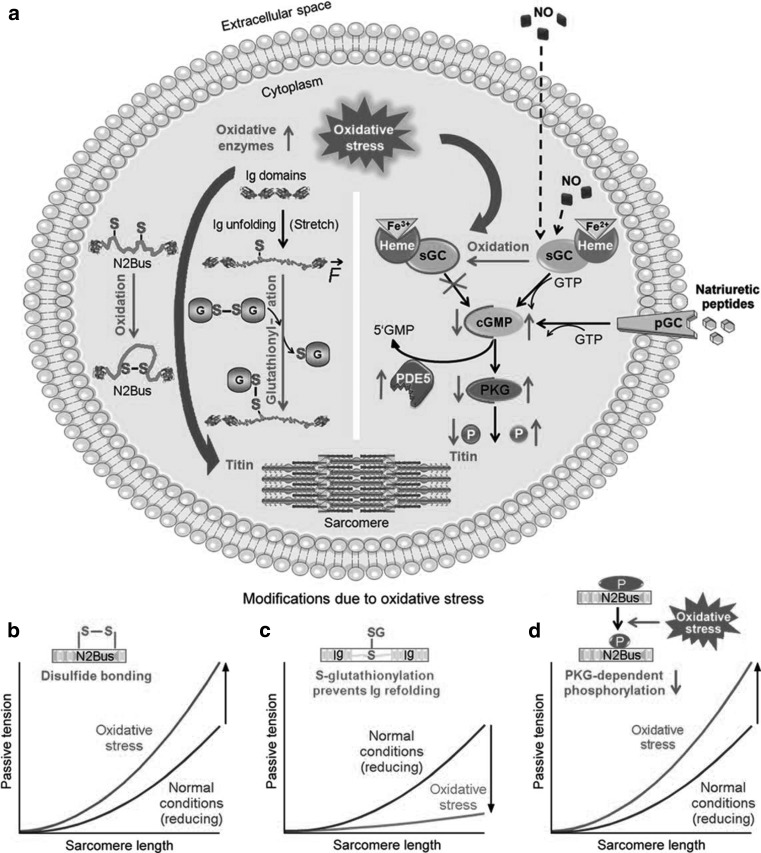
Fig. 4.
The effects of oxidative stress on titin and cardiomyocyte-based stiffness. a Oxidative stress induces post-translational modifications of titin, such as oxidation of cysteines in N2B-unique sequence of titin (N2-Bus) causing disulphide bonding (far left), S-glutathionylation of cysteines in unfolded Ig domains inhibiting domain refolding (left-middle), and reduced cGMP-dependent protein kinase-G (PKG)–dependent N2-Bus phosphorylation, because of oxidation of the haeme moiety in soluble guanylyl cyclase (sGC) and the ensuing blockade of cGMP production (right). Graphs in B to D show oxidative stress–related effects on titin-based passive tension caused by S–S bonding within N2-Bus (b), S-glutathionylation of unfolded titin-Ig domains (c), or depressed cGMP-PKG pathway activation (d). 5′GMP guanosine-5′-monophosphate, cGMP cyclic guanosine monophosphate, G glutathione, GSSG, glutathione-disulphide, NO, nitric oxide, P, titin phosphorylation, PDE5, phosphodiesterase-5, pGC, particulate guanylyl cyclase, PKG, cGMP-dependent protein kinase-G, and sGC, soluble guanylyl cyclase. (Used with permission from [62])