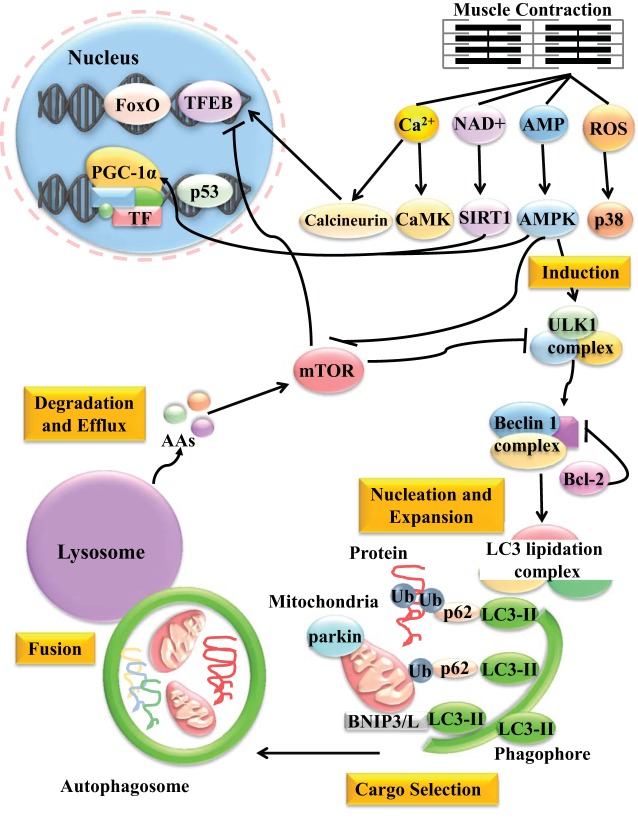
Fig. 1.
Exercise-induced autophagy signaling. During muscle contraction, levels of calcium, NAD+, AMP, and ROS increase. These molecular messengers activate their respective downstream effectors, CaMK/calcineurin, SIRT1, AMPK, and p38. These, in turn, initiate both acute autophagy and potentiate a longer term transcriptional program. AMPK can lead to the activation of the autophagy induction complex by ULK1 phosphorylation, as well as inhibition of mTOR, which normally acts to block autophagy. Autophagy induction is followed by nucleation and expansion of the autophagosome mediated by the beclin-1 complex and the lipidation of LC3 to LC3-II by a series of conjugation reactions by a series of autophagy-related proteins we termed the LC3 lipidation complex. The beclin-1 complex can also be antagonized by Bcl-2. During the cargo selection process, dysfunctional organelles (such as mitochondria) or protein aggregates are tagged for degradation by ubiquitin (Ub) and are subsequently recognized by the adaptor protein p62, which binds to Ub and coalesces the materials to be degraded into the growing autophagosome by interacting with LC3. Mitochondria can be tagged for degradation by ubiquitination of substrates on the outer mitochondrial membrane by the E3 Ub ligase parkin, or by mitochondria-specific receptors BNIP3/L, independent of Ub. The precise mechanism of cargo selection following exercise is yet to be elucidated. Once the cargo is engulfed, the mature autophagosome is sealed and delivered to the lysosome. The two then fuse together, and the contents are degraded by various hydrolases within the lysosomal lumen. Basic building blocks, such as animo acids (AAs) are then released and can negatively feed back on the pathway by reactivating mTOR. Long-term potentiation of an autophagy transcriptional pathway also occurs with exercise. Calcineurin can dephosphorylate the master regulator of the autophagy-lysosome system, TFEB, and allow its localization into the nucleus. p38 and ROS can also induce a p53-mediated transcriptional program, although that is yet to be confirmed. AMPK, p38, and SIRT1 can also activate FoxO-dependent gene transcription. Moreover, CaMK and calcineurin induce PGC-1α expression, while SIRT1, AMPK, and p38 upregulate PGC-1α activity. Once active in the nucleus, PGC-1α co-activates transcription factors, resulting in the increased expression of autophagy and lysosomal genes. See text for definition of acronyms.