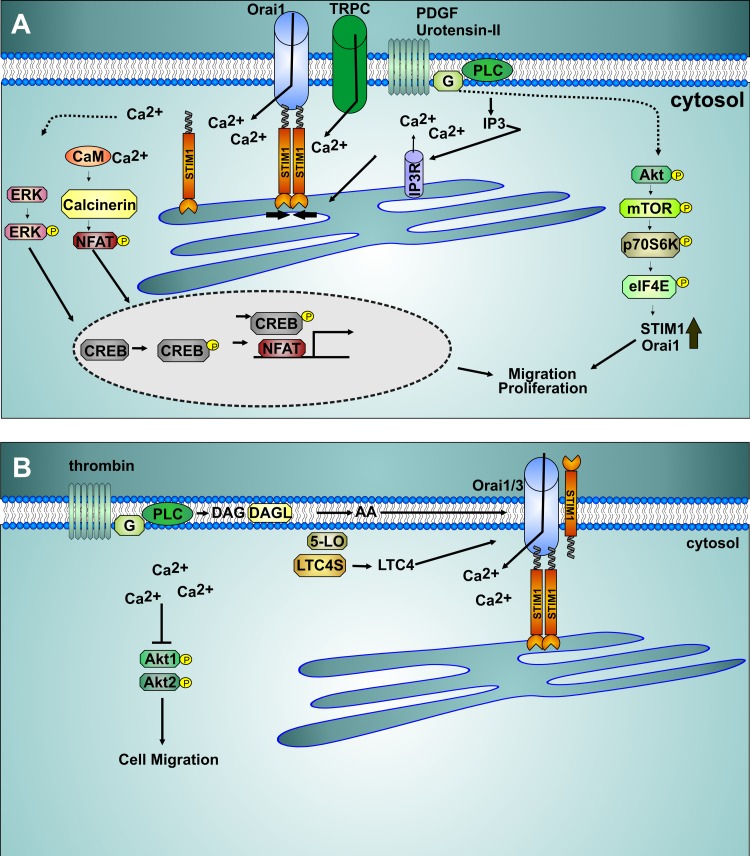
Fig. 2.
Store-dependent and -independent Ca2+ entry pathways in smooth muscle. A: activation of plasma membrane phospholipase C (PLC)-coupled receptors initiates production of inositol 1,4,5-triphosphate (IP3), promoting Ca2+ release and endoplasmic reticulum store depletion. Store depletion causes stromal-interacting molecule 1 (STIM1) translocation and binding to Orai1 Ca2+ channel to activate Ca2+ entry. Transient receptor potential canonical (TRPC) channel isoforms are also concomitantly activated in response to receptor stimulation in smooth muscle. Ca2+ entry activates the phosphatase calcinerin, with subsequent dephosphorylation and nuclear translocation of nuclear factor of activated T cells (NFAT), leading to transcription of genes involved in migration and proliferation. Store-operated Ca2+ entry (SOCE) was proposed to promote ERK and cAMP response element-binding protein (CREB) phosphorylation, enhancing CREB smooth muscle cell proliferation. Receptor activation of the Akt/mammalian target of rapamycin (mTOR)/P70S6 kinase (P70S6K)/eukaryotic translation initiation factor 4E (eIF4E) pathway promotes enhanced expression of STIM1 and Orai1 and enhanced SOCE. CaM, calmodulin; IP3R, IP3 receptor; G, G protein. B: receptor activation can lead to arachidonic acid (AA) generation through several routes, including metabolism of diacylglycerol (DAG) generated through PLC by DAG lipase. AA is further metabolized into downstream metabolites, including leukotrienes. Leukotriene C4 (LTC4) and AA activate Ca2+-selective channels composed of Orai1, Orai3, and STIM1, and this activation is independent of store depletion. Inhibition of these store-independent Orai1/Orai3 channels caused enhanced Akt1 and Akt2 phosphorylation and inhibited vascular smooth muscle cell migration. LTC4S, LTC4 synthase; 5-LO, 5-lipoxygenase.