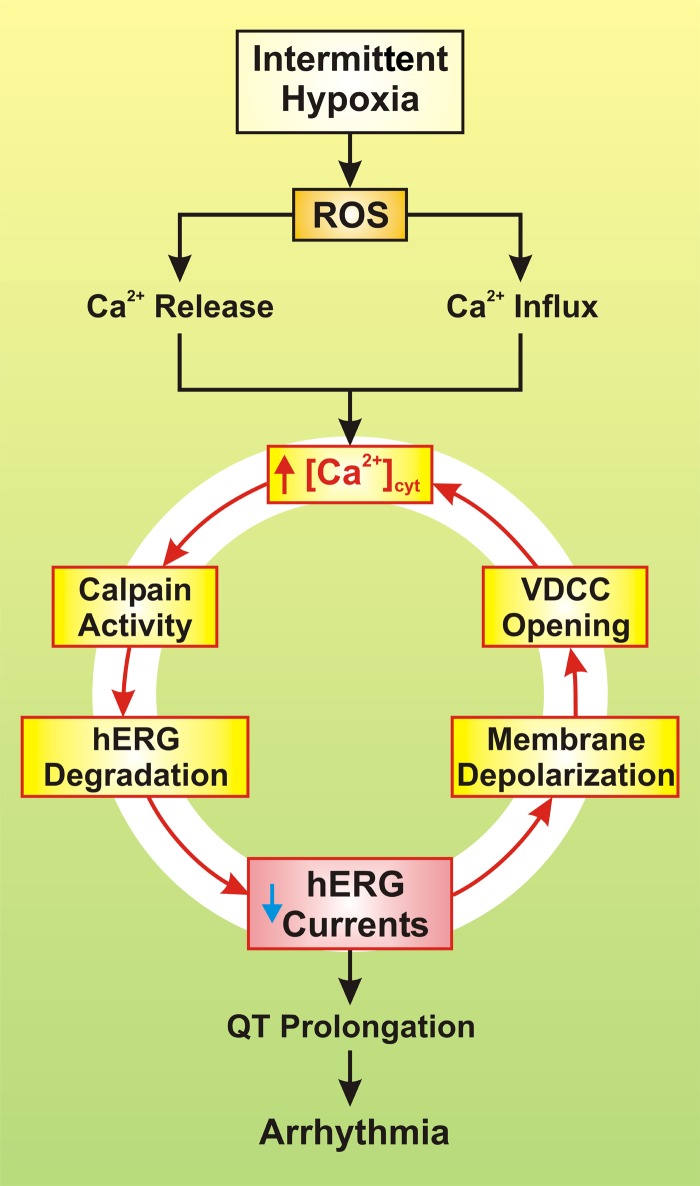
Fig. 1.
Proposed mechanisms involved in intermittent hypoxia-mediated arrhythmia. Reactive oxygen species (ROS), increased by intermittent hypoxia, trigger Ca2+ release from the sarcoplasmic reticulum (SR) and mitochondria and causes Ca2+ influx through voltage-dependent Ca2+ channels (VDCC) and transient receptor potential vanilloid (TRPV) channels. The resulting increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) then activates calpain, a Ca2+-dependent, non-lysosomal cysteine protease, which causes degradation of human ERG (hERG) channels and decrease in whole cell K+ currents through hERG channels. Reduced hERG K+ current then leads to prolongation of the Q-T interval and ultimately to arrhythmia and sudden cardiac death. The reduced hERG K+ current also causes membrane depolarization that opens VDCC, enhances Ca2+ influx through VDCC, and further increases [Ca2+]cyt in the cardiomyocytes. The loop formed by the initial ROS-mediated increase in [Ca2+]cyt and the calpain-mediated decrease in whole cell hERG K+ currents may serve as a pathogenic sequence of events for ROS-mediated arrhythmia in patients with OSA or individuals exposed to intermittent hypoxia.