In this multicenter study of patients who developed histoplasmosis on tumor necrosis factor-α blocker therapy, concomitant corticosteroid use and higher urine Histoplasma antigen levels were found to be independent predictors of severe disease. Outcomes were generally favorable with a 3.2% mortality rate.
Keywords: histoplasmosis, infliximab, adalimumab, etanercept, immune reconstitution syndrome
Abstract
Background. Histoplasmosis may complicate tumor necrosis factor (TNF)–α blocker therapy. Published case series provide limited guidance on disease management. We sought to determine the need for long-term antifungal therapy and the safety of resuming TNF-α blocker therapy after successful treatment of histoplasmosis.
Methods. We conducted a multicenter retrospective review of 98 patients diagnosed with histoplasmosis between January 2000 and June 2011. Multivariate logistic regression was used to evaluate risk factors for severe disease.
Results. The most commonly used biologic agent was infliximab (67.3%). Concomitant corticosteroid use (odds ratio [OR], 3.94 [95% confidence interval {CI}, 1.06–14.60]) and higher urine Histoplasma antigen levels (OR, 1.14 [95% CI, 1.03–1.25]) were found to be independent predictors of severe disease. Forty-six (47.4%) patients were initially treated with an amphotericin B formulation for a median duration of 2 weeks. Azole treatment was given for a median of 12 months. TNF-α blocker therapy was initially discontinued in 95 of 98 (96.9%) patients and later resumed in 25 of 74 (33.8%) patients at a median of 12 months (range, 1–69 months). The recurrence rate was 3.2% at a median follow-up period of 32 months. Of the 3 patients with recurrence, 2 had restarted TNF-α blocker therapy, 1 of whom died. Mortality rate was 3.2%.
Conclusions. In this study, disease outcomes were generally favorable. Discontinuation of antifungal treatment after clinical response and an appropriate duration of therapy, probably at least 12 months, appears safe if pharmacologic immunosuppression has been held. Resumption of TNF-α blocker therapy also appears safe, assuming that the initial antifungal therapy was administered for 12 months.
Tumor necrosis factor (TNF)–α blocker therapy has revolutionized the treatment of various autoimmune disorders. Approved TNF-α blockers include anti-TNF chimeric monoclonal antibodies (eg, infliximab, adalimumab) and the soluble TNF-α receptor etanercept. Histoplasmosis may complicate their use because TNF-α plays a critical role in the host's immune response to Histoplasma capsulatum [1–3]. The prevalence of infectious complications is higher in patients receiving monoclonal antibodies compared with those receiving the soluble TNF-α receptor [4]. This probably reflects differences in the mechanism of action and kinetics between the 2 drug classes [5].
Since the introduction of TNF-α blockers into clinical practice, postmarketing reports of histoplasmosis in endemic areas have been published [6]. The incidence of histoplasmosis is estimated to be 18.78 per 100 000 persons treated with infliximab and 2.65 per 100 000 persons treated with etanercept [7]. Published case series have limited information regarding clinical characteristics and disease outcome [8–11]. Regarding management of histoplasmosis in this setting, it is uncertain whether long-term, suppressive, antifungal therapy to prevent relapse should be continued after resolution of disease manifestations. TNF-α blocker therapy is usually discontinued after the diagnosis of an invasive fungal infection. Reducing immunosuppression may result in an immune reconstitution inflammatory syndrome (IRIS). Uncertainties remain with respect to the management of immunosuppression in IRIS. It also remains unclear whether biologic therapy can be safely reinstituted after successful treatment of histoplasmosis. Herein, we report our findings from a multicenter study on histoplasmosis associated with the use of TNF-α blockers.
METHODS
Study Cohort
We conducted a retrospective review study of patients who developed histoplasmosis as a complication of TNF-α blocker therapy. We included data on patients diagnosed at 20 US medical centers between 1 January 2000 and 30 June 2011. Most centers were located in endemic areas, and their investigators have collaborated in prior multicenter studies of histoplasmosis. Five centers were selected after correspondence with the main investigators about individual cases. Cases were identified through a search of medical records and laboratory/microbiology databases of the participating institutions as well as laboratory records of MiraVista Diagnostics (Indianapolis, Indiana). The study was approved by the institutional review boards at major participating centers or patients provided consent to be included in the study. A few cases from the Mayo Clinic [9], Indiana University [8], and Nationwide Children's Hospital [12] have been previously published.
Patients had clinical signs and symptoms consistent with the diagnosis of histoplasmosis (eg, fever, weight loss, respiratory or gastrointestinal manifestations, lymphadenopathy, hepatosplenomegaly) and fulfilled at least 1 of the following criteria: (1) growth of H. capsulatum from clinical specimens; (2) histopathologic or cytopathologic demonstration of morphologic forms consistent with H. capsulatum from any biopsy tissue; (3) urine or serum positive for Histoplasma antigen via enzyme-linked immunoassay; or (4) positive Histoplasma serology using immunodiffusion methodology with detection of H or M bands and/or complement fixation at a titer of ≥1:8.
Disseminated histoplasmosis was defined as the presence of clinical, microbiologic, or radiographic evidence of extrapulmonary involvement. Diagnosis of pulmonary histoplasmosis required respiratory symptoms and radiographic findings of infiltrates and/or mediastinal lymphadenopathy in the absence of evidence of disseminated disease. Histoplasmosis was classified as mild if hospitalization was not necessary, moderate if hospitalization was required at the time of diagnosis, and severe if patients required initial management in an intensive care unit. Patients were classified as having IRIS if all 3 of the following criteria were fulfilled: (1) new appearance or worsening of clinical or radiographic manifestations consistent with an inflammatory process or histopathology showing granulomatous lesions, (2) symptoms that could not be explained by a newly acquired infection, and (3) negative culture results and/or reduced Histoplasma antigen levels [13].
Diagnostic Studies
Specimens were tested with the Histoplasma antigen enzyme-linked immunoassay at MiraVista Diagnostics. Prior to May 2007, specimens were tested with a semiquantitative assay. Specimens that were received or available after May 2007 were tested with the newer-generation quantitative assay [14]. The latter assay permits quantification below the level of 0.6 ng/mL, which is arbitrarily assigned a value of 0.5 ng/mL. All serum (but not urine) specimens were pretreated with 4% ethylenediaminetetraacetate acid (EDTA) at 100°C for 6 minutes to allow for dissociation of immune complexes [15]. Results of other diagnostic tests (eg, culture, cytopathology, histopathology, and serology) were performed at the originating institutions or other commercial laboratories.
Statistical Analysis
Descriptive statistics were used to summarize the cohort. For categorical variables, comparisons were made using the χ2 or Fisher exact test, as appropriate. For continuous variables that were not normally distributed, the Wilcoxon rank-sum or Kruskal–Wallis test was used. Histoplasma antigen testing performed with the newer-generation assay was included in the analysis. Concentrations above the level of quantification (>19.0 ng/mL) were assigned a value of 19. Logistic regression was used to evaluate risk factors for severe disease. Odds ratios with 95% confidence intervals were determined for each risk factor. Factors found to be associated with severe disease at the .10 significance level in univariate analysis were included in a multivariate model. A backward stepwise approach was used for the modeling. The model fit was tested using the Hosmer and Lemeshow goodness-of-fit test. Statistical software Stata/SE, version 13.1 (StataCorp, College Station, Texas) was used.
RESULTS
Clinical Characteristics
We identified 98 cases of histoplasmosis. The majority of the cases were diagnosed at 2 centers—38 at the Mayo Clinic and 19 at Indiana University. Demographic and clinical characteristics are shown in Table 1. Rheumatoid arthritis was the most common underlying disease and infliximab the most commonly used immunosuppressive agent. A risk factor analysis is presented in Table 2. Underlying autoimmune disease and type of TNF-α blocker (infliximab/adalimumab vs etanercept) were not found to be associated with severe disease. In multivariate analysis, concomitant corticosteroid use and higher urine antigen levels were found to be the only independent predictors of disease severity.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | No. (%) |
|---|---|
| Age, y, mean (range) | 45 (9–84) |
| Female | 56 (57.1) |
| Race | |
| White | 74 (97.4) |
| Black | 1 (1.3) |
| Asian | 1 (1.3) |
| Unknown | 22 |
| Underlying disordera | |
| Rheumatoid arthritis (including juvenile RA) | 52 (53.1) |
| Inflammatory bowel disease | 37 (38.1) |
| Psoriasis | 7 (7.2) |
| Ankylosing spondylitis | 2 (2.1) |
| Takayasu arteritis | 1 (1.0) |
| Sarcoidosis | 1 (1.0) |
| Uveitis | 1 (1.0) |
| TNF-α blocker used | |
| Infliximab | 66 (67.3) |
| Adalimumab | 23 (23.5) |
| Etanercept | 9 (9.2) |
| Additional immunosuppressants | |
| Methotrexate | 43 (43.9) |
| Corticosteroids | 33 (33.7) |
| Azathioprine | 13 (13.3) |
| 6-mercaptopurine | 6 (6.1) |
| Leflunomide | 1 (1.0) |
| 2 additional immunosuppressants | 28 (28.6) |
| Time (months) to diagnosis after TNF-α blocker initiation, median (range) | 15.5 (1–88) |
| Organ involvement | |
| Disseminated disease | 74 (75.5) |
| Pulmonary involvement | 78 (79.6) |
| Fungemia | 18 (18.4) |
| Liver | 15 (15.3) |
| Spleen | 15 (15.3) |
| Bone marrow | 14 (14.3) |
| Gastrointestinal | 12 (12.2) |
| Lymph nodes | 5 (7.6) |
| Arthritis | 4 (4.1) |
| Skin | 3 (3.1) |
| Central nervous system | 2 (2.0) |
| Adrenal | 2 (2.0) |
| Sinus | 1 (1.0) |
| Epiglottis | 1 (1.0) |
| Disease severity | |
| Mild | 26 (26.5) |
| Moderate | 55 (56.1) |
| Severe | 17 (17.3) |
Abbreviations: RA, rheumatoid arthritis; TNF, tumor necrosis factor.
a Three subjects had 2 concurrent autoimmune disorders.
Table 2.
Risk Factors for Severe Disease
| Parameter | Mild/Moderate Disease (n = 81) | Severe Disease (n = 17) | Univariate OR (95% CI) | P Value | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Age, y, mean (range) | 44 (9–84) | 51 (18–76) | 1.02 (.99–1.05) | .16 | ||
| Male sex | 35 (43.2) | 7 (41.2) | 0.92 (.32–2.66) | .88 | ||
| Underlying disease | ||||||
| Rheumatoid arthritis | 42 (51.9) | 10 (58.8) | 1.33 (.46–3.83) | .6 | ||
| Inflammatory bowel disease | 33 (40.7) | 4 (23.5) | 0.45 (.13–1.49) | .19 | ||
| Psoriasis | 7 (8.6) | 1 (5.9) | 0.66 (.76–5.75) | .71 | ||
| Immunosuppression | ||||||
| Infliximab/adalimumab | 72 (88.9) | 17 (100) | 3.60 (1.22–10.61) | .35 | ||
| Corticosteroid therapy | 23 (28.4) | 10 (58.8) | 3.21 (.85–12.05) | .02 | 3.94 (1.06–14.60) | .04 |
| Cytotoxic therapy | 48 (59.3) | 14 (82.3) | .08 | … | ||
| Fungemia | 9 (11.1) | 7 (41.2) | 5.60 (1.71–18.38) | .005 | … | |
| Urine antigen, ng/mL, mean (SD)a | 7.69 (6.52) | 13.76 (7.45) | 1.14 (1.04–1.25) | .006 | 1.14 (1.03–1.25) | .008 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
a Urine antigen ranged from <0.6 to >19 ng/mL in both groups.
Diagnostic Studies
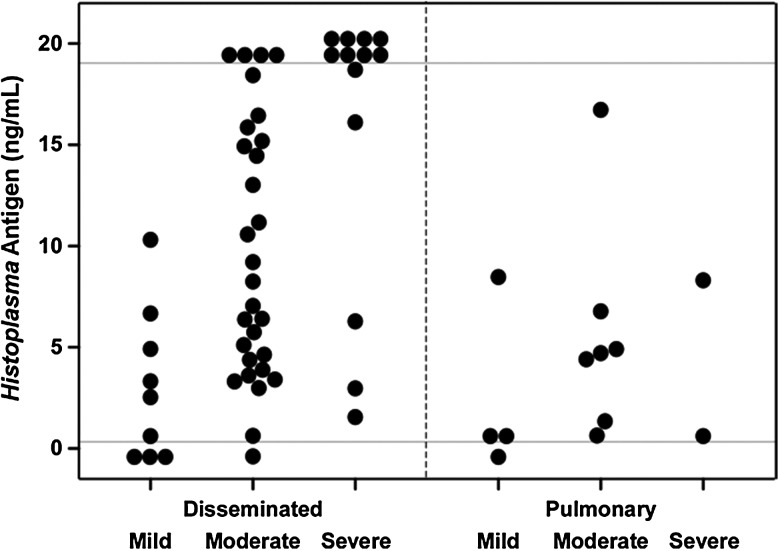
Diagnostic test results are presented in Table 3. Histoplasma capsulatum was isolated in culture in 41 of 73 (56.2%) patients. Overall, Histoplasma antigen was detected in 72 of 82 (87.8%) patients (71 of 81 had positive urine antigen, 14 of 18 had positive serum antigen; in a single patient, serum but not urine antigen was tested and found to be positive). Mean urine antigen level was 9.70 ng/mL (median, 8.24) for disseminated disease and 3.81 ng/mL (median, 0.99) for disease limited to the lungs (P = .008). Mean serum antigen was 9.95 ng/mL (median, 2.88) for disseminated disease. Serum antigen was tested in 1 patient with disease limited to the lungs; the result was negative. Urine antigen test results in relation to disease severity are shown in Figure 1. Serology was positive in 65 of 74 (87.8%) patients. Pathology results for any site were positive in 39 of 63 (61.9%) patients.
Table 3.
Comparison of Diagnostic Tests in Disseminated and Pulmonary Cases
| Diagnostic Test | All Cases (N = 98) | Disseminated Cases (n = 74) | Pulmonary Cases (n = 24) | P Value (Disseminated vs Pulmonary) |
|---|---|---|---|---|
| Culture (any site) | 41/73 (56.2) | 33/56 (58.9) | 8/17 (47.1) | .39 |
| Blood culture | 18/33 (54.5) | 18/30 (60.0) | 0/3 (0) | NA |
| BAL culture | 19/26 (73.1) | 13/18 (72.2) | 6/8 (75.0) | 1.0 |
| Urine antigen positivity | 71/81 (87.7) | 55/62 (88.7) | 16/19 (84.2) | .69 |
| Urine antigen levela | 8.54 ± 7.26 | 9.70 ± 7.30 | 3.81 ± 4.90 | .008 |
| Serum antigen positivity | 14/18 (77.8) | 14/17 (82.4) | 0/1 | .22 |
| Serum antigen levela | 9.40 ± 13.99 | 9.95 ± 14.21 | ||
| Serology | 65/74 (87.8) | 46/54 (85.2) | 19/20 (95.0) | .43 |
| Immunodiffusion | 50/65 (76.9) | 34/48 (70.8) | 16/18 (88.9) | .20 |
| Complement fixation | 55/66 (83.3) | 36/45 (80.0) | 18/20 (90.0) | .48 |
| Pathologyb (any site) | 39/63 (61.9) | 35/50 (70) | 4/13 (30.8) | .01 |
| Pathology (lung biopsy) | 12/22 (54.5) | 9/12 (75) | 3/10 (30.0) | .08 |
| BAL cytopathology | 8/11 (72.7) | 8/11 (72.7) | None sent | NA |
Data are presented as No. of patients with positive test results/No. of patients tested (%) unless otherwise specified. The diagnosis was established by antigen detection alone in 8 subjects, and by serology alone in 13 subjects.
Abbreviations: BAL, bronchoalveolar lavage fluid; NA, not applicable; SD, standard deviation.
a Mean antigen concentration ± SD in ng/mL.
b Pathology sources: Lung biopsy in 12 of 22 (54.4%) patients; BAL cytopathology in 8 of 11 (72.7%) patients; gastrointestinal biopsy in 9 of 9 (100%) patients; bone marrow biopsy in 7 of 13 (53.8%) patients; liver biopsy in 3 of 3 (100%) patients; and peripheral lymph node biopsy in 2 of 4 (50%) patients.
Figure 1.
Urine Histoplasma antigen levels (third-generation assay) at diagnosis, by disease severity and site of involvement. Each closed circle represents an individual patient. Results below the lower gray horizontal line (cutoff for positivity for the assay) were classified as negative. Results above the upper gray horizontal line represent specimens with positive results >19.0 ng/mL, which is the upper limit for quantification of the assay.
Antifungal Treatment
Forty-six of 97 (47.4%) patients were initially treated with various formulations of amphotericin B. Forty-one of 74 (55.4%) patients with disseminated disease and 5 of 24 (20.8%) with disease limited to the lungs received amphotericin B (P = .003). Median duration of treatment with amphotericin B preparations was 2 weeks (range, 2 days to 20 weeks). All patients initially treated with amphotericin B received an azole preparation as step-down therapy with a median duration of 12 months (range, 2–43 months).
Fifty of 97 (51.5%) patients were initially treated with an azole. Overall, 91 patients were treated with itraconazole and 5 with voriconazole. Because of low serum concentrations or adverse events, itraconazole was changed to fluconazole in 4 patients and to voriconazole in 3. Azole treatment was given for a median duration of 12 months (range, 3–27 months). Thirty-four of 82 (41.5%) patients received treatment for >12 months.
Only 1 patient did not receive antifungal therapy. This patient was taking infliximab and methotrexate for rheumatoid arthritis. He experienced respiratory failure, and imaging demonstrated a worsening nodular infiltrate. Open lung biopsy showed granulomatous inflammation, and culture grew H. capsulatum. Urine Histoplasma antigen was <0.6 ng/mL. The patient was treated with corticosteroids, and the other immunosuppressants were discontinued. The patient improved symptomatically, and the infiltrate resolved almost completely without any antifungal therapy 2 months later. At last follow-up 5 years later, there was no clinical evidence of recurrence.
Management of Immunosuppression
TNF-α blocker therapy was initially discontinued in 96.9% of patients (95/98). In 2 of them, therapy was discontinued 3 months prior to the diagnosis of histoplasmosis; we included these cases as their disease was associated with the use of biologic therapy, even though diagnosis was delayed. TNF-α blockers were continued along with antifungal therapy in 3 patients (Table 4).
Table 4.
Clinical Findings of Patients Who Were Continued on Tumor Necrosis Factor–α Blocker Therapy After the Diagnosis of Histoplasmosis
| Age, Sex, Disease | TNF-α Blocker, Duration Prior to Histoplasmosis | Diagnosis | Extent of Disease | Antifungal Treatment, Duration | Outcome | Follow-up Duration |
|---|---|---|---|---|---|---|
| 55 F Psoriasis | Etanercept, 82 mo | Positive serology | Mild, pulmonary | Itraconazole, 7 mo | Remission | 50 mo |
| 21 F Crohn disease | Infliximab, 30 mo | Positive BAL culture, serology | Mild, pulmonary | Itraconazole, 10 mo | Remission | 70 mo |
| 13 M Crohn disease | Adalimumab, 16 mo | Positive serology | Mild, cervical lymphadenopathy | Itraconazole, 6 mo | Remission | 6 mo |
None of these patients was receiving other immunosuppressive therapy. Histoplasma antigen testing was not performed.
Abbreviations: BAL, bronchoalveolar lavage fluid; TNF, tumor necrosis factor.
TNF-α blocker therapy was resumed in 33.8% of patients (25/74 where data was available) at a median time of 12 months (range, 1–69 months) after the diagnosis of histoplasmosis. These patients received antifungal treatment for a median of 11 months (range, 3–27 months). In 7 of these 25 patients, antifungal treatment was discontinued at a median time of 20 months (range, 2–60 months) prior to restarting the TNF-α blocker; in 1 patient, itraconazole was used as prophylaxis to prevent relapse after restarting infliximab. In 3 patients, the TNF-α blocker was resumed along with discontinuation of the antifungal. In 5 patients, TNF-α blocker therapy was resumed before stopping the antifungal. Exact timing of treatment modification was not available for the remaining patients. Histoplasma urine antigen was available for 7 patients around the time that antifungal treatment was discontinued—1 patient had an undetectable level, 3 had a value of <0.6 ng/mL, and 3 had a value of ≥1 ng/mL. Of those with a value of <0.6 ng/mL, 1 had recurrent disease 39 months after resuming TNF-α blocker therapy and died, as described below. All others had undetectable levels and no clinical evidence of recurrence at last follow-up despite receiving TNF-α blocker therapy off antifungal prophylaxis.
Methotrexate or azathioprine/6-mercaptopurine was discontinued in 47 patients. Doses were continued unchanged in 11. Corticosteroid treatment was discontinued in 7 patients, and the corticosteroid dose was decreased in 11, increased in 2, and continued unchanged in 10 patients.
Disease Outcome
Patients were followed for a median time of 32 months (range, 1–120 months). Outcome data are not available on 3 patients. There was no evidence of recurrent disease based on either clinical findings or increase in Histoplasma antigen in 94.7% (90/95) of patients by the end of the follow-up period. Two patients with severe disseminated disease died within 4 weeks of diagnosis. Their urine antigen levels at diagnosis were 18.7 ng/mL and >19.0 ng/mL, respectively. Three (3.2%) patients had recurrent disease (Table 5). Two of them recovered uneventfully, but the other received only 6 months of itraconazole and did well for several years after reinstituting TNF-α blocker therapy off antifungal treatment, with negative antigen tests, only to develop fulminant and fatal histoplasmosis 39 months after the initial episode. Urine antigen was negative following initial therapy and 3 years prior to recurrence, but was detectable at <0.6 ng/mL 4 months prior to the recurrence.
Table 5.
Clinical Characteristics and Laboratory Findings of Patients Who Had Recurrent Disease
| Age, Sex, Disease | TNF-α Blocker, Duration Prior to Diagnosis | Diagnosis | Extent of Disease at Diagnosis | Treatment, Duration | TNF-α Blocker Resumed | Time to Recurrence | Diagnosis of Recurrence | Treatment of Recurrence, Outcome |
|---|---|---|---|---|---|---|---|---|
| 33 M Rheumatoid arthritis | Adalimumab, 27 mo | Positive BAL culture, lung granuloma, Urine Ag: 16.72 |
Moderate, pulmonary | Itraconazole for 8 mo, Urine Ag not retested prior to relapse | Yes at 6 mo | 8 mo, on antifungal | Fatigue, diaphoresis Urine Ag: 38.59 |
L-AmB, recovered |
| 54 F Rheumatoid arthritis | Adalimumab, unknown | Hepatic granuloma, Urine Ag: 13.01 | Moderate, disseminated | L-AmB for 4 wk, then voriconazolea for 6 mo Urine Ag: 2.17 at 12 mo |
No | 14 mo, off antifungal | Fever, cough Urine Ag: 4.76 |
Voriconazole, recovered |
| 33 F Crohn disease | Infliximab, 40 mo | Positive serology, Urine Ag: 3.26 | Mild, disseminated | Itraconazole, 6 mo Urine Ag: negative at 9 mo |
Yes at 6 mo | 39 mo, off antifungal | Fulminant sepsis, Urine Ag: >19 | L-AmB, died |
TNF-α blocker was initially discontinued in all patients. Antigen levels are in ng/mL.
Abbreviations: Ag, antigen; BAL, bronchoalveolar lavage fluid; L-amB, liposomal amphotericin B; TNF, tumor necrosis factor.
a The patient did not tolerate itraconazole.
At the end of treatment, quantitative urine Histoplasma antigen levels were available for 33 patients. Mean value was 0.75 ng/mL (range, 0–6.4 ng/mL). Histoplasma antigen was undetectable in 14 patients and measured <0.6–0.9 ng/mL in 13 patients. Urine antigen was 1.0–3.9 ng/mL in 4 patients and ≥4.0 ng/mL in 2 patients; none of them relapsed. At the end of the follow-up period, quantitative antigen was available in 58 patients who did not have clinical evidence of disease and did not experience relapse. Mean urine antigen level was 0.53 ng/mL (range, 0–7.77 ng/mL). Urine antigen was undetectable in 39 patients, <0.6–0.9 ng/mL in 12 patients, 1.0–3.9 ng/mL in 3 patients, and ≥4.0 ng/mL in 4 patients.
Immune Reconstitution Inflammatory Syndrome
Nine (9.2%) patients were considered to have IRIS (Table 6). The syndrome manifested with worsening respiratory symptoms, adult respiratory distress syndrome, worsening lymphadenopathy, liver function abnormalities, or cervical lymphadenopathy. Most patients had received prior treatment with infliximab and had developed disseminated histoplasmosis. Median time to onset of IRIS after TNF-α blocker discontinuation was 6 weeks (range, 1–45 weeks). Three patients diagnosed with IRIS were treated with corticosteroids along with antifungal therapy. There were no deaths, and all patients recovered uneventfully.
Table 6.
Clinical Characteristics, Treatment, and Outcome of Patients With Possible Immune Reconstitution Inflammatory Syndrome
| Study Code | Age, Sex, Disease | TNF-α Blocker Treatment |
Antifungal Treatment |
IRIS Manifestation | Culture, Urine Antigen | Treatment Modification | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Drug | Time From Discontinuation to Onset of IRIS | Drug | Duration Prior to Onset of IRIS | ||||||
| 1a | 66 F Sarcoidosis | Infliximab | 10 wk | AMB deoxycholate | 7 d | ARDS, reticulonodular infiltrates evolving to bilateral consolidation | 8.48 U at diagnosis 4.39 U at IRIS |
Corticosteroid | Recovered after 40 d hospitalization |
| 2a | 47 M Rheumatoid arthritis | Adalimumab | 6 wk | Voriconazole | 33 d | Worsening liver function tests | 5.55 U at diagnosis 1.77 U at IRIS |
Voriconazole changed to fluconazole | Recovered |
| 4a | 9 F Juvenile rheumatoid arthritis | Etanercept | 1 wk | Liposomal AMB | 4 d | Worsening respiratory failure | 5.08 ng/mL at diagnosis <0.6 ng/mL at IRIS |
None | Recovered |
| 10a | 50 M Rheumatoid arthritis | Infliximab | 4 wk | AMB deoxycholate | 7 d | ARDS, reticulonodular infiltrates evolving to bilateral consolidation | BAL culture positive at presentation Undetectable at IRIS |
None | Recovered after 6 wk of mechanical ventilation |
| 11a | 62 M Crohn disease | Infliximab | 10 wk | AMB | 1 d | ARDS, reactive hemophagocytic syndrome | BAL and bone marrow culture positive | Corticosteroid | Recovered after 12 d of mechanical ventilation and 40 d hospitalization |
| 12a | 46 F Crohn disease | Infliximab | 9 wk | Itraconazole | 0 | Enlarging lung mass and hilar lymphadenopathy | FNA of lymph node showed reactive inflammation, Ag 4.6 U | None | Recovered |
| 14a | 20 F Crohn disease | Infliximab | 6 wk | Itraconazole | 6 d | ARDS, reticulonodular infiltrates evolving to bilateral consolidation | 5.72 ng/mL at diagnosis, 5.21 ng/mL at IRIS | Itraconazole changed to AMB | Recovered after 1 wk mechanical ventilation and 20 d hospitalization |
| 19a | 29 F Crohn disease | Adalimumab | 4 wk | Itraconazole | 12 d | New low-density splenic lesions, hilar lymphadenopathy and pulmonary nodules | 10.3 ng/mL at diagnosis, 4.29 ng/mL at IRIS | None | Recovered |
| 20 | 58 F Rheumatoid arthritis | Etanercept | 45 wk | Itraconazole | 45 wk | Left supraclavicular lymphadenopathy with biopsy showing granulomatous inflammation and fungal elements | 8.46 ng/mL at diagnosis, undetectable at IRIS. Tissue culture no growth | Continued etanercept, prednisone, itraconazole | Recovered |
Abbreviations: Ag, antigen; AMB, amphotericin B; ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage fluid; FNA, fine needle aspiration; IRIS, immune reconstitution inflammatory syndrome; TNF, tumor necrosis factor.
a Previously published [8].
DISCUSSION
Herein, we report our findings from a multicenter retrospective study of histoplasmosis complicating TNF-α blocker therapy. Pulmonary involvement was prominent and most patients had disseminated disease at diagnosis. Unusual extrapulmonary manifestations such as joint or skin involvement were encountered, similar to patients developing tuberculosis as a complication of TNF-α blocker therapy [16]. The vast majority of the patients were white, which may reflect the lower prevalence of autoimmune conditions among nonwhites, or possibly racial disparities in access to immunosuppressive therapy.
To establish the diagnosis in a timely fashion, familiarity with diagnostic assays is necessary. In a previous multicenter study involving both immunocompetent and immunosuppressed individuals, urine antigen was detected in 91.8% and serum antigen in 100% of patients with disseminated histoplasmosis [17]. In the current study, antigenuria was present in 88.7% and antigenemia in 82.4% of patients with disseminated disease. Antigen levels were significantly higher in patients with severe disease at presentation. Notably, urine antigen levels among patients with disease limited to the lungs in this study were higher than previously reported [17, 18]. We demonstrated that 87.8% of those who had antibody tests performed tested positive either by immunodiffusion or complement fixation. Among solid organ transplant (SOT) recipients, antibody testing has been reported to be positive in only 36% [18]. This likely reflects the different type of immunosuppression used in organ transplant.
Routine serologic screening for histoplasmosis prior to initiation of TNF-α blocker therapy is generally not recommended, and we did not collect information about such testing. Antifungal treatment is indicated in all patients developing histoplasmosis associated with TNF-α blocker therapy. In our study, patients with mild disease who were started on azole treatment had a favorable outcome. Ideally, as physicians become more aware of this entity, patients should be diagnosed at an earlier stage and receive treatment before the disease progresses. One patient with pulmonary disease did not receive antifungal treatment and recovered only by reducing immunosuppression. The low antigen concentration supports a low fungal burden inconsistent with severe disease. These findings suggest that the illness was caused by an inflammatory response, and gradually subsided during continued corticosteroid therapy. This approach of withholding antifungal therapy cannot be generally recommended.
The role of lifelong suppressive antifungal therapy to prevent relapse is uncertain. A firm recommendation about the need for chronic suppressive therapy among immunosuppressed transplant recipients was not provided in the Infectious Diseases Society of America histoplasmosis treatment guidelines [19]. In our cohort, the vast majority of patients who were symptom-free at completion of treatment did not have evidence of recurrence by the end of the follow-up period. This finding supports the safety of discontinuing antifungals in patients for whom TNF-α blocker therapy is not resumed, after treatment with at least 12 months of an appropriate antifungal agent. Of note, a small number of patients had detectable antigen levels after successful completion of antifungal treatment without evidence of recurrence on follow-up.
Resuming TNF-α blocker therapy, when clinically indicated, may be considered in individuals treated for histoplasmosis who have no evidence of residual disease and undetectable antigen levels. The patient who received a 6-month course of antifungal treatment and developed fatal disease shortly after resurgence of low-level antigenuria demonstrates the importance of close monitoring for clinical or laboratory evidence of recurrent disease after reinstitution of immunosuppressive treatment. We recommend that such monitoring occur every 3 months. In select cases, TNF-α blocker therapy can be continued even after the diagnosis of histoplasmosis, along with concomitant antifungal therapy, as demonstrated by the favorable outcome of 3 such patients with mild disease in this study. Nonetheless, the vast majority of patients had TNF-α blocker therapy discontinued, and we consider this to be the standard of care.
In this study, we found a mortality rate of 3.2%. The reported higher mortality rate of 10% among SOT recipients with histoplasmosis [18] is likely explained by their net state of immunosuppression. IRIS, a state of imbalance between protective immunity and inflammatory pathology [20], was diagnosed in 9.2%. Besides discontinuation of immunosuppressive treatment, the immunomodulatory characteristics of antifungal drugs may contribute to IRIS. More specifically, amphotericin B deoxycholate upregulates the transcription of inflammatory cytokines [21]. In the presence of worsening clinical manifestations, decreasing Histoplasma antigen levels suggest IRIS, whereas increasing levels suggest disease progression or relapse.
Our study has several limitations. Most cases were diagnosed at the Mayo Clinic, a major referral center located in the Midwest, and Indiana University Hospitals located in Indianapolis, a hyperendemic area. Milder forms of disease were frequently identified at these 2 sites and were possibly missed at centers where histoplasmosis is less commonly considered. However, when we included center as a variable in the logistic regression model to predict disease severity, it did not reach statistical significance (P = .12). Onset of disease in relation to initiation of TNF-α blocker treatment could not be assessed, and this is another limitation. In the comparison of diagnostic tests, we included specimens that were tested for Histoplasma antigen with the quantitative assay and not the previously used semiquantitative assay. We do not have information on the type of TNF-α blocker that was used upon resumption of immunosuppression. A small number of patients was lost to follow-up, and the number of recurrent cases was small (only 3); hence, firm conclusions on disease recurrence cannot be drawn. Despite these limitations, this is the largest published study to date on histoplasmosis complicating TNF-α blocker therapy with follow-up clinical and laboratory data.
In conclusion, with the increasing use of TNF-α blockers for a variety of autoimmune disorders, healthcare providers should be able to recognize, diagnose, and treat histoplasmosis, especially in endemic areas. Discontinuation of antifungal treatment after a favorable clinical response and an appropriate duration of therapy, probably at least 12 months, appears to be safe if pharmacologic immunosuppression has been held. Resumption of TNF-α blocker therapy also appears to be safe with or without antifungal therapy, assuming that the initial antifungal therapy was administered for at least 12 months. If TNF-α blocker therapy is continued or resumed, close clinical monitoring is strongly advised.
Notes
Acknowledgments. The authors thank Seo Young Park, PhD, for assistance with statistical analysis and Tia Gore for carefully reviewing the manuscript. We also thank the participating centers (contributed cases shown in parentheses): Mayo Clinic, Rochester, Minnesota (38); Indiana University, Indianapolis (19); Cleveland Clinic, Cleveland, Ohio (8); Nationwide Children's Hospital, Columbus, Ohio (5); Kansas University, Wichita (5); University of Kansas Medical Center, Kansas City (5); Wright State University, Dayton, Ohio (2); University of Nebraska Medical Center, Omaha (2); Infectious Disease Associates of Kansas City, Missouri (2); University of Kentucky, Lexington (2); Meritus Medical Center, Hagerstown, Maryland (1); University of Wisconsin, Madison (1); Long Island Infectious Disease Associates, Huntington, New York (1); University of Michigan Medical Center, Ann Arbor (1); Kaiser Permanente, Fontana, California (1); Ellis Hospital, Schenectady, New York (1); University of Iowa, Iowa City (1); Samaritan Infectious Disease, Corvallis, Oregon (1); Wright-Patterson Medical Center, Dayton, Ohio (1); and Health Ball Memorial Hospital, Muncie, Indiana (1).
Financial support. P. V. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health http://dx.doi.org/10.13039/100000002 (award number KL2TR000146).
Potential conflicts of interest. R. K. A. receives research support for multicenter clinical studies from Viropharma/Shire, Astellas, Chimerix, and Merck. L. J. W. is President of MiraVista Diagnostics, the laboratory that performs Histoplasma antigen testing. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol 1998; 160:6072–82. [PubMed] [Google Scholar]
- 2.Wood KL, Hage CA, Knox KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J Respir Crit Care Med 2003; 167:1279–82. [DOI] [PubMed] [Google Scholar]
- 3.Deepe GS., Jr Modulation of infection with Histoplasma capsulatum by inhibition of tumor necrosis factor-alpha activity. Clin Infect Dis 2005; 41(suppl 3):S204–7. [DOI] [PubMed] [Google Scholar]
- 4.Toh S, Li L, Harrold LR, et al. Comparative safety of infliximab and etanercept on the risk of serious infections: does the association vary by patient characteristics? Pharmacoepidemiol Drug Saf 2012; 21:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furst DE, Wallis R, Broder M, Beenhouwer DO. Tumor necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin Arthritis Rheum 2006; 36:159–67. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–70. [DOI] [PubMed] [Google Scholar]
- 7.Wallis RS, Broder M, Wong J, Lee A, Hoq L. Reactivation of latent granulomatous infections by infliximab. Clin Infect Dis 2005; 41(suppl 3):S194–8. [DOI] [PubMed] [Google Scholar]
- 8.Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Joseph Wheat L. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis 2010; 50:85–92. [DOI] [PubMed] [Google Scholar]
- 9.Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EL. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009. BMC Infect Dis 2011; 11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc 2008; 83:181–94. [PubMed] [Google Scholar]
- 11.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–5. [DOI] [PubMed] [Google Scholar]
- 12.Dotson JL, Crandall W, Mousa H, et al. Presentation and outcome of histoplasmosis in pediatric inflammatory bowel disease patients treated with antitumor necrosis factor alpha therapy: a case series. Inflamm Bowel Dis 2011; 17:56–61. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis 2007; 7:395–401. [DOI] [PubMed] [Google Scholar]
- 14.Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol 2007; 14:1587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swartzentruber S, LeMonte A, Witt J, et al. Improved detection of Histoplasma antigenemia following dissociation of immune complexes. Clin Vaccine Immunol 2009; 16:320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol 2006; 2:602–10. [DOI] [PubMed] [Google Scholar]
- 17.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53:448–54. [DOI] [PubMed] [Google Scholar]
- 18.Assi M, Martin S, Wheat LJ, et al. Histoplasmosis after solid organ transplant. Clin Infect Dis 2013; 57:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 20.Gupta AO, Singh N. Immune reconstitution syndrome and fungal infections. Curr Opin Infect Dis 2011; 24:527–33. [DOI] [PubMed] [Google Scholar]
- 21.Bellocchio S, Gaziano R, Bozza S, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother 2005; 55:214–22. [DOI] [PubMed] [Google Scholar]