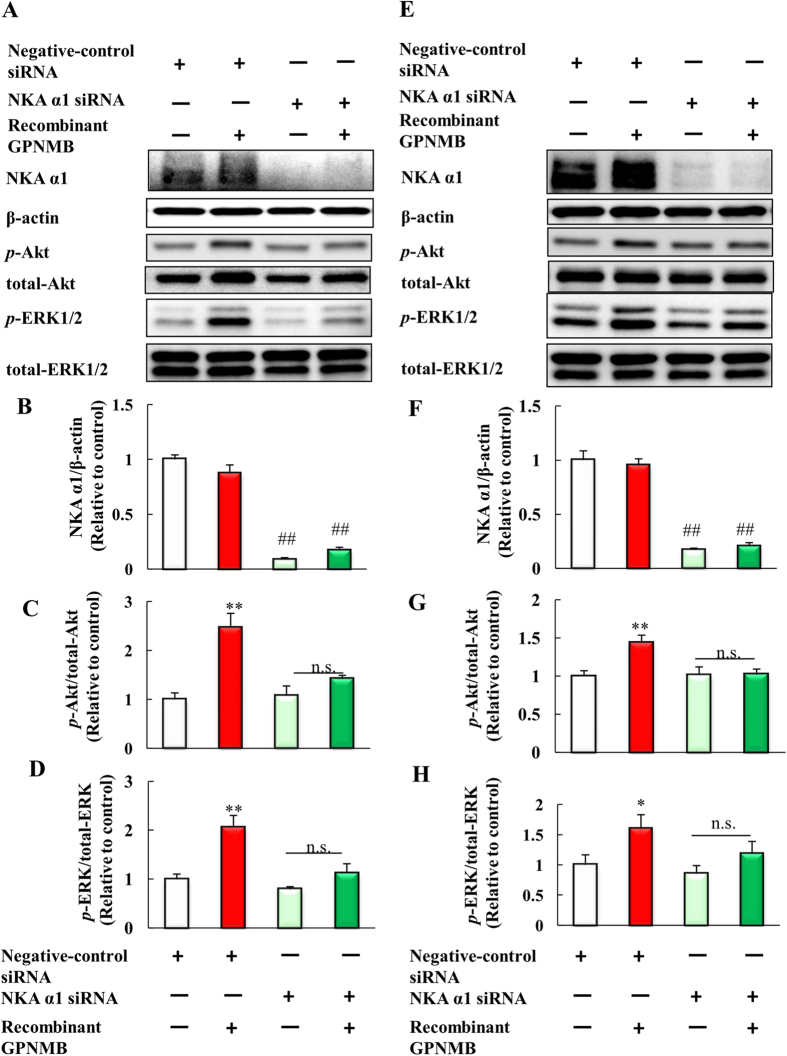
Figure 7. Activation of the PI3K/Akt and MEK/ERK pathways by the extracellular fragment of GPNMB was blocked by NKA α1 siRNA.
(A) NSC34 cells were transfected with mouse NKA α1 siRNA or negative-control siRNA, and then treated with the human recombinant GPNMB (2.5 μg/ml). Western blotting analyses of NKA α1, β-actin, phosphorylated-Akt, total-Akt, phosphorylated-ERK1/2, and total-ERK1/2. (B) The protein levels of NKA α1 were quantified relative to β-actin. (C) The protein levels of phosphorylated-Akt were quantified relative to total-Akt. (D) The protein levels of phosphorylated-ERK1/2 were quantified relative to total-ERK1/2. Each column represents the mean ± S.E.M. (n = 4). n.s.: not significant. ##p < 0.01 vs. negative-control, **p < 0.01 vs. GPNMB treated group (Student’s t-test). (E) 661W cells were plated at 1.5 × 104 cells/well in 24-well plates and incubated overnight. Negative-control siRNA and NKA α1 siRNA transfected for 24 h, and then cells were treated with PBS or human recombinant GPNMB. Western blotting analyses of NKA α1, β-actin, phosphorylated-Akt, total-Akt, phosphorylated-ERK1/2, and total-ERK1/2. (F) The protein levels of NKA α1 were quantified relative to β-actin. (G) The protein levels of phosphorylated-Akt were quantified relative to total-Akt. (H) The protein levels of phosphorylated-ERK1/2 were quantified relative to total-ERK1/2. Each column represents the mean ± S.E.M. (n = 4 or 5). n.s.: not significant. ##p < 0.01 vs. negative-control, *p < 0.05, **p < 0.01 vs. GPNMB treated group (Student’s t-test).