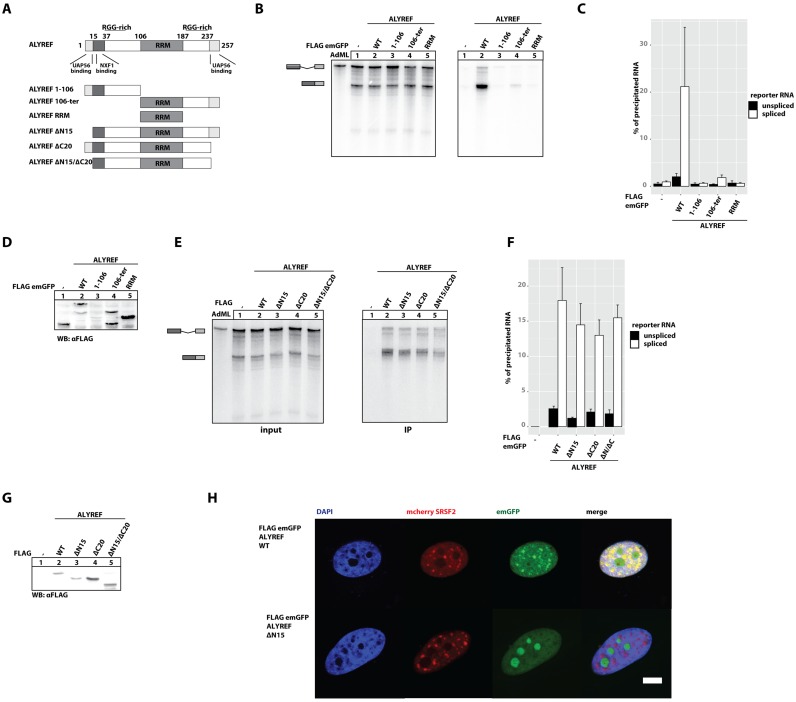
Figure 3.
ALYREF binding to RNA in vitro is not mediated by its canonical RNA binding domain and is independent of UAP56 binding. (A) Schematic representation of ALYREF wild-type and mutants used in the study. (B) In vitro splicing reactions of AdML RNA were supplemented with extracts expressing FLAG-emGFP ALYREF truncation mutants (as in Figure 1A). Schematic representations of splicing and digestions products are depicted on the left side of the autoradiograph. (C) The results of three, independent, biological repetitions were quantified. Band intensities corresponding to the unspliced reporter RNA and spliced RNA are presented as black and white bars, respectively. (D) Expression of FLAG-tagged proteins in HEK 293 extracts used in (B) was determined by immunoblot analysis using a FLAG-antibody. (E) In vitro splicing reactions of AdML RNA were supplemented with extracts expressing FLAG-emGFP ALYREF truncation mutants as in (B). Schematic representations of splicing and digestions products are depicted on the left side of the autoradiograph. (F) The results of three independent biological repetitions were quantified and presented on the graph. Band intensities corresponding to the unspliced reporter RNA and spliced RNA are presented as black and white bars, respectively. (G) Expression of FLAG-tagged proteins in HEK 293 extracts used in (E) was determined by immunoblot analysis using a FLAG-antibody. (H) Localization of FLAG-emGFP ALYREF wild-type and mutant in HeLa cells. DAPI was used to stain nuclear DNA. Scale bar, 5 μm.