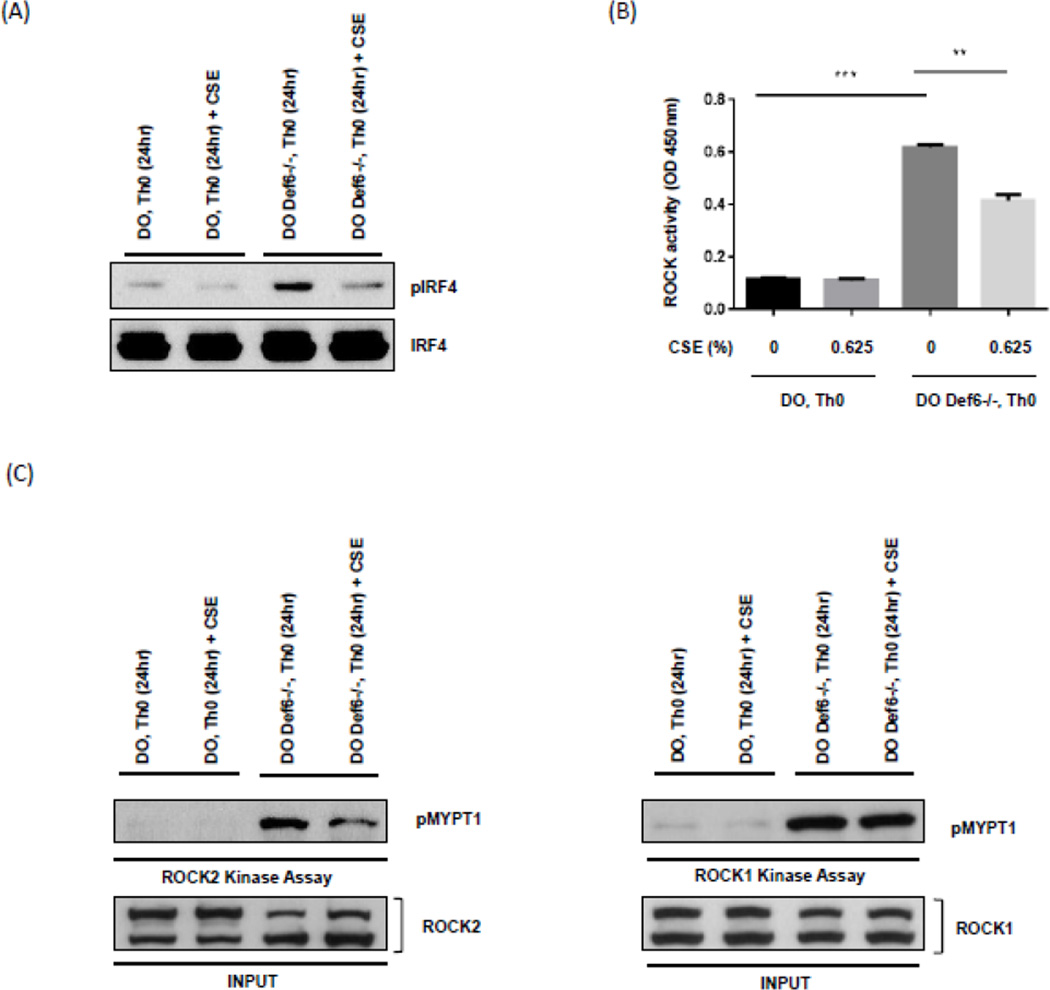
Fig. 2.
Exposure of Def6−/− DO11.10 CD4+ T cells to cigarette smoke extract (CSE) inhibits IRF4 phosphorylation and ROCK2 kinase activity. CD4+ T cells from either Def6+/+ DO11.10 (DO Th0) or Def6−/− DO11.10 (DO Def6−/− Th0) mice were purified and cultured under neutral (Th0) (αCD3 2µg/ml, αCD28 1µg/ml) conditions for 3 days and then rested for 4 days in IL-2 (20ng/ml). After resting, CD4+ T cells were restimulated under neutral (Th0) conditions in the presence or absence of sterile CSE (0.625%) for 24 hours. (A) Nuclear extracts from each condition were assayed for IRF4 phosphorylation by Western blotting using an antibody directed against phosphorylated IRF4 (pIRF4) (upper panel). Total IRF4 levels were evaluated by reprobing with an antibody against total IRF4 (lower panel). (B) Total ROCK kinase activity in whole cell extracts from each condition was assessed by an ELISA-based assay. (C) ROCK2 (left) and ROCK1 (right) kinase activity was examined by incubating immunoprecipitated ROCK2 or ROCK1 with purified recombinant MYPT1 (rMYPT1) as substrate. Phosphorylated rMYPT1 (pMYPT1) (shown in upper panel) was then detected using anti-phospho-MYPT1 antibody. Total ROCK2 or ROCK1 levels for each sample are shown in the lower panel. Data are representatives of two independent experiments. Statistical analyses were performed by unpaired Student’s t test; ns, P ≥ 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.