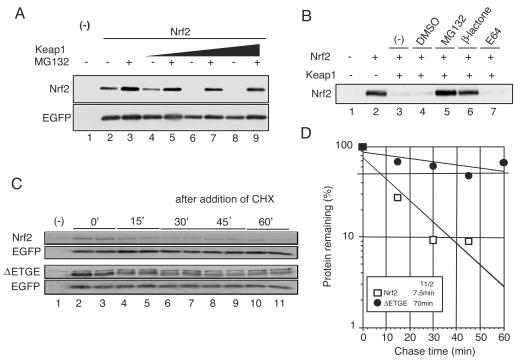
FIG. 1.
Assay system to examine the degradation mechanism of Nrf2. (A) Keap1 promotes Nrf2 degradation in the in vivo degradation system. An Nrf2 expression vector (2 μg) was transfected into Cos7 cells (90% confluent) with or without the Keap1 expression vector (1.5 μg). At 24 h after transfection, the cells were treated with dimethyl sulfoxide (DMSO) (lanes 1, 2, 4, 6, and 8) and 2 μM MG132 (lanes 3, 5, 7, and 9) for 12 h and directly lysed in sodium dodecyl sulfate sample buffer. (Upper panel) Whole-cell extracts were subjected to immunoblot analysis with an anti-Nrf2 antibody. (Lower panel). The expression level of cotransfected EGFP was used as an internal control. (B) Proteasome-specific inhibitors stabilize the Nrf2 protein. Transfected cells were treated with DMSO (lane 4), 2 μM MG132 (lane 5), 2 μM clasto-lactacystin β-lactone (lane 6), and E64 (lane 7) for 12 h. Immunoblot analysis was performed as described above. (C and D) The Nrf2 expressed in this system was rapidly degraded in a Keap1-dependent manner. Nrf2 and ΔETGE mutant were transfected into cells along with Keap1. At 36 h after transfection, the cells were treated with 10 μM cycloheximide (CHX) per ml for the periods indicated. (Upper panel) Whole-cell extracts were subjected to immunoblot analysis with an anti-Nrf2 antibody. (Lower panel). The expression level of EGFP was used as an internal control. The averages of the relative band intensities of Nrf2 (open squares) and ΔETGE mutant (closed circles) represent two independent experiments performed in duplicate.