Table 1.
Optimization of coupling conditions.[a]
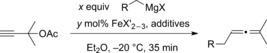
| R | Equiv | Iron cat. | mol % of cat. | Additive | Yield [%][a] |
|---|---|---|---|---|---|
| Ph[b] | 1.25 | none | – | – | 0 |
| 1.25 | [Fe(acac)2] | 5 | – | 63 | |
| 1.25 | [Fe(acac)3] | 5 | – | 94 | |
| 1.5 | [Fe(acac)3] | 5 | – | 93 | |
| 1.25 | FeCl3 | 5 | – | 82 | |
| 1.25 | [Fe(acac)3] | 5 | NMP[e] | 53 | |
| 1.25 | [Fe(acac)3] | 5 | TMEDA[e] | 25 | |
| Bn[c] | 1.25 | none | – | – | 0 |
| 1.25 | [Fe(acac)2] | 5 | – | 50 | |
| 1.25 | [Fe(acac)3] | 1 | – | 66 | |
| 2.5 | [Fe(acac)3] | 1 | – | 67 | |
| 1.25[d] | [Fe(acac)3] | 1 | – | 47 | |
| 1.25 | [Fe(acac)3] | 1 | NMP[e] | 49 |
[a] Reaction conditions: 0.2 m solution of propargyl acetate (1 mmol) in Et2O with dropwise addition of Grignard reagent during 5 min. Yield was determined by NMR spectroscopy. [b] X=Cl. [c] X=Br. [d] Addition during 30 sec. [e] Used 0.5 equivalents of additive. acac=acetylacetonate.
