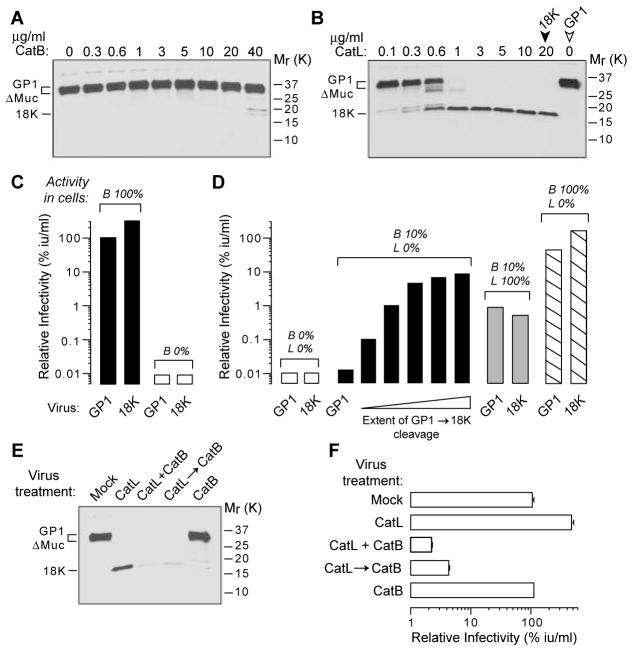
Fig. 2.
Endosomal cysteine proteases act directly on EboV GPΔMuc to mediate infection of Vero cells. (A and B) Purified CatB (A) and CatL (B) cleave GPΔMuc to GP118K. VSV-GPΔMuc was incubated with enzyme for 1 hour at pH 5.5 and 37°C. Mock-treated VSV particles containing GP1 (open arrowhead) and CatL-treated VSV particles containing GP118K (18K) (solid arrowhead) were used in (C) and (D). (C) VSV particles containing GP118K are highly infectious and fully dependent on cellular CatB activity. Cells were not treated (solid bars) or pretreated with E-64d (300 μM) (open bars) to inactivate CatB. Approximate CatB activity (B%) in these cells is indicated above the bars. (D) VSV particles containing GP118K bypass a block to GP1 cleavage within cells. Cells were treated with inhibitors to obtain the approximate levels of cellular CatB (B%) and CatL (L%) activity shown (also see Fig. 1 and table S1). Open bars, 300 μM E-64d; solid black bars, 10 μM FYdmk; solid gray bars, 40 μM CA074; striped bars, 1 μM FYdmk. Cells were then infected with VSV-GPΔMuc containing GP1 only, GP118K only, or increasing amounts of GP118K (wedge) (generated by incubation with increasing concentrations of CatL for 1 hour at pH 5.5 and 37°C). (E) Purified CatB efficiently digests CatL-derived GP118K. VSV-GPΔMuc was incubated with the indicated enzymes for 1 hour at pH 5.5 and 37°C {CatB, 40 μg/ml; CatL, 20 μg/ml; CatB and CatL together (CatB + CatL); or CatL followed by CatB [CatL→CatB (30 min each)]}. (F) Digestion of CatL-derived GP118K by CatB inactivates VSV-GPΔMuc. Infectivities of VSV particles from (E) are shown. Averages of two replicates are shown in (C) and (D) and are representative of three independent experiments. Error bars, SD from three replicates; Mr, relative molecular weight in kilodaltons (K).