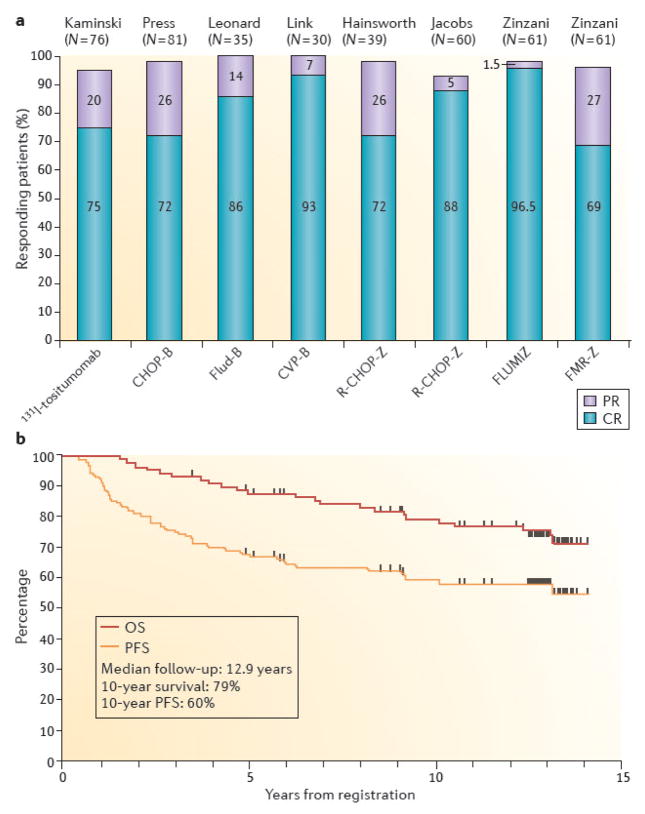
Figure 3. Results of selected trials of radioimmunotherapy as part of front-line therapy for follicular lymphoma.
(A) Overall response rates, partial remission rates (purple), and complete remission rates (blue) are indicated for eight studies utilizing: 131I-tositumomab alone3; cyclophosphamide, doxorubicin, vincristine, and prednisone followed by 131I-tositumomab (CHOP-B)60; fludarabine followed by 131I-tositumomab (Flud-B)61; cyclophosphamide, vincristine, and prednisone followed by 131I-tositumomab (CVP-B)67; rituximab plus CHOP followed by 90Y-ibritumomab tiuxetan (R-CHOP-Z)129; fludarabine plus mitoxantrone followed by 90Y-ibritumomab tiuxetan (FLUM IZ)64; or fludarabine, mitoxantrone, rituximab, and zevalin followed by 90Y-ibritumomab tiuxetan (FMR-Z)130. Data are graphed from the published studies. (B) Progression-free and overall survival of 90 eligible patients with advanced Follicular Non-Hodgkin’s Lymphoma treated with six cycles of CHOP chemotherapy followed by tositumomab/131I-tositumomab on SWOG protocol S9911.