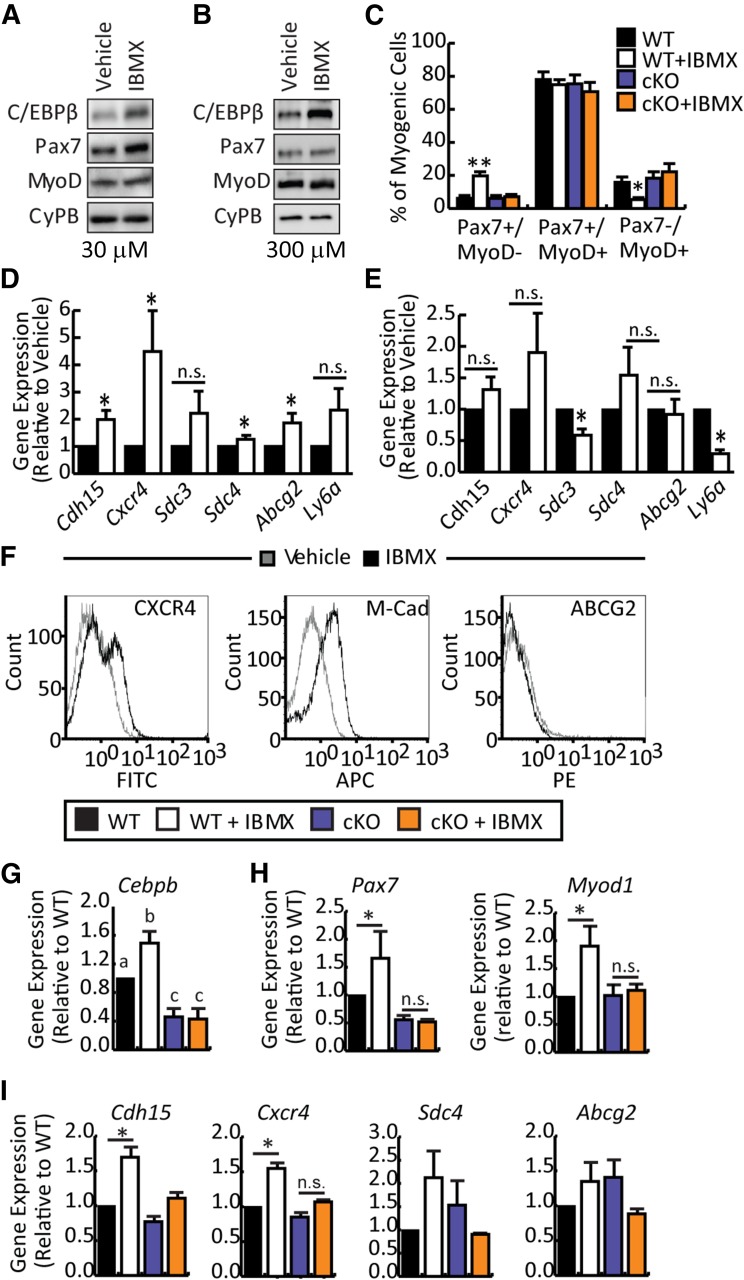
Figure 3.
IBMX-mediated stimulation of satellite cell marker expression and migration is dependent on C/EBPβ. (A, B): Primary myoblasts were cultured in vehicle or IBMX (30 and 300 μM) for 5 days and analyzed by Western blot for C/EBPβ, Pax7, and MyoD protein expression. Cyclophilin B was a loading control. (C): Percentage of quiescent (Pax7+/MyoD−), proliferating (Pax7+/MyoD+), and differentiating (Pax7−/MyoD+) cells as determined by immunocytochemistry in primary myoblast cultures treated for 5 days in GM with 30 μM IBMX or vehicle. (D): Real-time quantitative polymerase chain reaction of satellite cell markers Cdh15, Cxcr4, Sdc3, Sdc4, Abcg2, and Ly6a in primary myoblasts cultured for 5 days in GM with 30 μM IBMX or vehicle. (E): Satellite cell marker expression in primary myoblasts cultured in GM for 5 days in the absence or presence of 300 μM IBMX. (F): Flow cytometry histograms of CXCR4,M-Cad, and ABCG2 expression on vehicle and IBMX-treated myoblasts. (G): Cebpb expression in myoblasts isolated from C/EBPβ cKO or WT non-Cre-expressing floxed littermates treated with vehicle or 30 μM IBMX for 5 days in GM. (H): Myogenic markers expression (Pax7 and Myod1) in myoblasts cultured as in (G). (I): Satellite cell marker expression (Cdh15, Cxcr4, Sdc4, and Abcg2) in myoblasts cultured as in (G). Data are presented as mean ± SEM (n ≥ 3; ∗∗, p < .01; ∗, p < .05; means with different letters have a p value <.05, and means indicated with the same letter are not statistically significantly different). Abbreviations: APC, allophycocyanin; C/EBPβ, CCAAT/enhancer-binding protein β; cKO, conditional knockout; CyPB, cyclophilin B; FITC, fluorescein isothiocyanate; IBMX, isobutylmethylxanthine; M-Cad, m-cadherin; n.s., not significant; WT, wild-type.