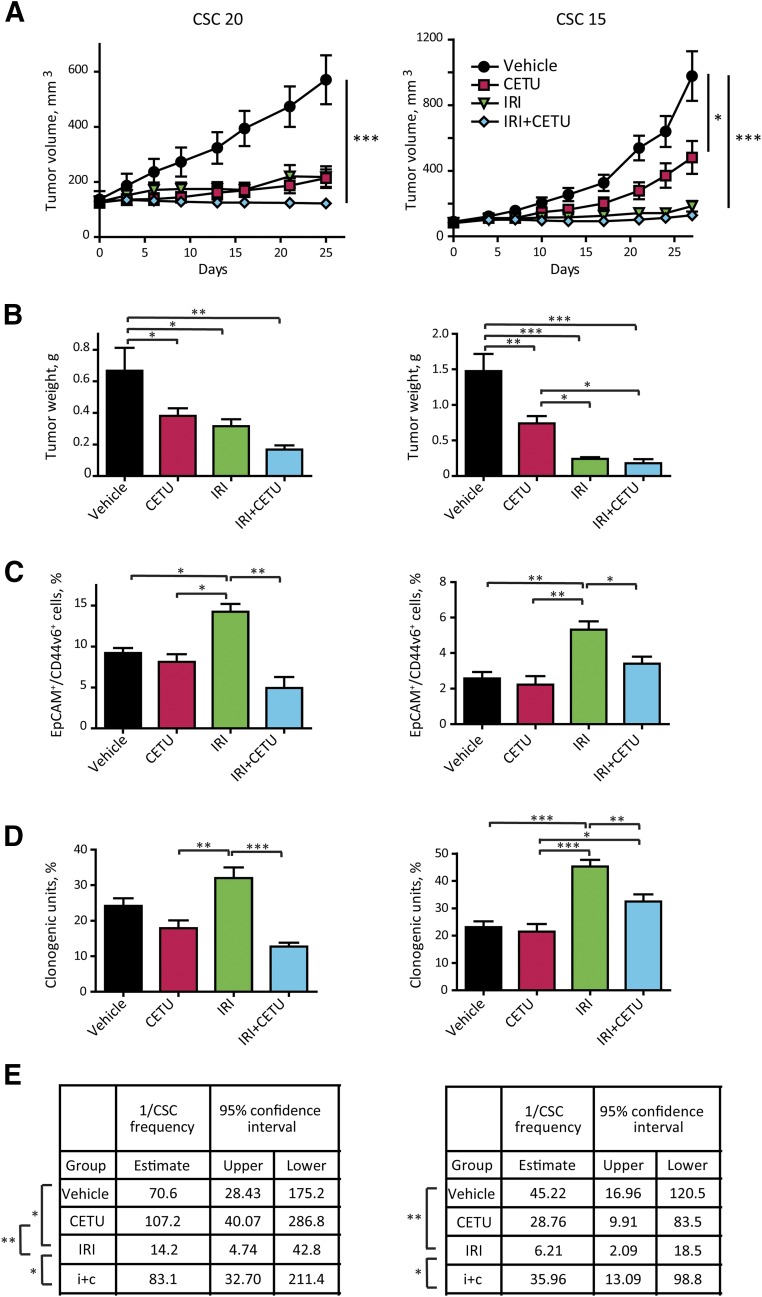
Figure 5.
Effect of epidermal growth factor receptor inhibition on the CSC compartment in vivo. (A): Effect of cetuximab and irinotecan on the growth of xenografts derived from highly cetuximab-responsive CSC 20 and medium-responsive CSC 15. After tumor establishment, mice were treated with either cetuximab (10 mg/kg intravenously twice weekly) or irinotecan (15 mg/kg intraperitoneally weekly) or their combination; control animals were treated with vehicle only. Data represent the average of 8–10 tumors ± SEM (B): Tumor weight at the time of excision. (C): Flow cytometry analysis for EpCAM/CD44v6 on cells dissociated from xenografts in (A). Data represent the average of six xenografts for group ± SD (D): Clonogenicity assay performed ex vivo on cells isolated from xenografts as in (A). Cells were plated in triplicate, at 500 cells/ml per dish as described in Materials and Methods. Colonies were scored at day 21. Data represent the average of six to eight xenografts per group ± SEM. Statistical significance in (A–D) was calculated with one-way analysis of variance. (E): Second transplantation of cells dissociated from xenografts of in (A). Cells dissociated from individual tumors (six tumors per treatment group) were retransplanted subcutaneously into secondary recipient mice. For each individual tumor, different cell doses were tested as explained in Materials and Methods. Mice were recorded as negative when no graft was observed after 24 weeks from the inoculation. CSC frequency was calculated with the extreme limiting dilution analysis software, as described in the Materials and Methods. Statistical significance of the results was calculated with chi-square analysis. ∗, p < .05, ∗∗, p < .01, ∗∗∗, p < .001. Abbreviations: CETU, cetuximab; CSC, cancer stem cell; IRI, irinotecan.