Abstract
Rationale
The effect of stem/progenitor cells on myocardial perfusion and clinical outcomes in patients with refractory angina (RFA) remains unclear because studies published to date have been small phase I-II trials.
Objective
We performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the effect of cell-based therapy in patients with RFA who were ineligible for coronary revascularization.
Methods and Results
Several data sources were searched from inception till September 2015, which yielded six studies. The outcomes pooled were indices of angina (anginal episodes, Canadian Cardiovascular Society [CCS] angina class, exercise tolerance, anti-anginal medications), myocardial perfusion, and clinical end-points. We combined the reported clinical outcomes (myocardial infarction, cardiac-related hospitalization, and mortality) into a composite end-point (MACE). Mean difference (MD), standardized mean differences (SMD), or odds ratio (OR) were calculated to assess relevant outcomes. Our analysis shows an improvement in anginal episodes (MD -7.81;95% CI, -15.22−-0.-41), use of anti-anginal medications (SMD -0.59;CI, -1.03−-0.14), CCS class (MD -0.58;CI, -1.00−-0.16), exercise tolerance (SMD 0.331;CI, 0.08−0.55), and myocardial perfusion (SMD -0.49;CI, -0.76−-0.21) and a decreased risk of MACE (OR 0.49;CI, 0.25−0.98) and arrhythmias (OR 0.25; 95% CI, 0.06−0.98) in cell-treated patients compared with patients on maximal medical therapy.
Conclusions
The present meta-analysis indicates that cell-based therapies are not only safe but also lead to an improvement in indices of angina, relevant clinical outcomes, and myocardial perfusion in patients with RFA. These encouraging results suggest that larger, phase III RCTs are in order to conclusively determine the effect of stem/progenitor cells in RFA.
Keywords: Refractory angina, cell-based therapy, stem cells, progenitor cells, meta-analysis, angina, perfusion
Subject Terms: Cell Therapy, Chronic ischemic heart disease, Metaanalysis
INTRODUCTION
The prevalence of refractory angina (RFA) in the United States is estimated between 600,000 and 1.8 million.1, 2 With the advances made in the management of coronary artery disease, prolonged survival, and an aging population, the incidence of debilitating angina refractory to medical therapy (also referred to as no-option angina) is increasing.3 These patients present a major clinical problem because they either are ineligible for revascularization or do not adequately benefit from it due to the presence of microvascular disease; no other effective treatment is available.4 RFA places a great burden on society not only because of recurrent hospitalization and resource use,5 but also because of disability and lost productivity. Therefore, many novel therapeutic options have been explored in this patient population, including enhanced external counterpulsation, transcutaneous electric nerve stimulation, and transmyocardial laser revascularization; however, the response to these approaches has been disappointing.6–8 Moreover, the majority of studies of novel therapeutic modalities for ischemic artery disease have been conducted in stable patients rather than in those refractory to medical therapy.9, 10
Cell-based therapies have generated considerable interest in the field of ischemic heart disease because of their potential to promote neovascularization and endothelial repair,8, 11–13 thereby improving myocardial perfusion. Although several studies have been conducted to assess the effect of cell therapy in patients with angina refractory to conventional medical therapy and ineligible for coronary revascularization,14–19 the small size of these phase I-II trials and the lack of uniform primary end-points make it difficult to discern an efficacy signal. As a result, the effect of stem/progenitor cells on myocardial perfusion and clinical outcomes in RFA remains unclear; there is no phase III trial underway to corroborate the findings of these proof-of-concept trials.20 A meta-analysis published in 2013 concluded that cell-based therapy is safe and effective in RFA.21 However, that study21 failed to evaluate all of the functional and clinical end-points examined in the individual studies; importantly, the impact of cell therapy on myocardial perfusion was not assessed. Here we present a comprehensive, current review and meta-analysis of the safety and efficacy of cell-based therapy in RFA that expands on the previous analysis by including a new study,19 by assessing all of the functional and clinical end-points measured, and by exploring the effect of cell therapy on myocardial perfusion.
METHODS
Data sources and search strategy
This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The search strategy and subsequent literature search were performed by an experienced medical reference librarian. The search strategies were developed in PubMed, and translated to match the subject headings and keywords for Embase, Cochrane Central Register of Controlled Trials, and ISI Web of Science from inception through September 8, 2015. In addition, conference proceedings were searched for articles pertaining to the search criteria. The following MeSH, Emtree and keyword search terms were used in combination: cardiac stem cell therapy, bone marrow derived mononuclear cells, cardiac progenitor cells, endothelial progenitor cells (EPCs), refractory angina, intractable angina, drug resistant angina, coronary heart disease, coronary perfusion, myocardial perfusion, myocardial ischemia, single photon emission computed tomography (SPECT), controlled trials, intervention study and randomized controlled trials. The search accounted for plurals and variations in spelling with the use of appropriate wildcards. To identify further articles, we hand-searched related citations in review articles and commentaries. All results were downloaded into EndNote (Thompson Reuters) and duplicate citations were identified and removed.
Study selection
Two investigators (TF, AP) independently assessed the eligibility of the studies identified. The studies that were evaluated were RCTs that focused on the role of cell therapy in patients with angina refractory to medical therapy who were not eligible for coronary revascularization.
Data extraction
From the included studies, two reviewers (ARK, AT) independently extracted data on the population under study, patient characteristics, type of cell-based therapy used, and relevant outcomes. The outcomes measured in our analysis were the safety and efficacy of cell-based therapy. The main efficacy outcomes studied were indices of angina, myocardial perfusion, and clinical end-points. Safety of cell-based therapy was measured by the adverse events reported in the included studies.
The indices of angina measured were changes in frequency of angina episodes, Canadian Cardiovascular Society (CCS) angina class, exercise tolerance, change in anti-anginal medications, and quality of life. Myocardial perfusion was determined by single photon emission computed tomography (SPECT), which was the imaging modality used in individual studies. Clinical end-points reported in the studies were myocardial infarction, cardiac-related hospitalization, and mortality. Because a small number of these outcomes were reported in a limited number of studies, and because none of the studies was powered to assess these clinical end-points, we combined the cardiovascular outcomes into a composite cardiac end-point (major adverse cardiac events, MACE) that includes myocardial infarction, cardiac-related hospitalization, and mortality.
Data synthesis and statistical analysis
Continuous data were reported either as mean (standard deviation) or median (inter-quartile range). If the data were reported as median, mean and standard deviation were estimated.22 For continuous variables, the mean change between end of follow-up and baseline was measured in both groups; the mean difference (MD) was calculated as the difference between the mean change in the cell-treated group and the mean change in the control group. The MD was used to estimate changes in frequency of angina episodes, CCS class, and use of anti-anginal medications. The standardized mean difference (SMD)(calculated in an analogous manner) was used to assess changes in myocardial perfusion and exercise tolerance, in which different units of measurements were used in different studies. For dichotomous variables, an odds ratio (OR) was calculated for MACE and occurrence of adverse events. Meta-analyses were performed with a fixed-effects model; a random-effects model was used if heterogeneity was encountered. The I2 statistic was used to assess heterogeneity among studies.23 Publication bias was assessed by means of a funnel plot; the Begg and Mazumdar test was used to assess funnel plot asymmetry and publication bias if needed.24 Sensitivity analysis was done to investigate the associated heterogeneity and the effect of individual studies on it.
Quality assessment
Two reviewers (ARK, AP) independently assessed the methodological quality of the selected studies using the Cochrane risk of bias tool. This scale is used to explore the adequacy of sequence generation, allocation sequence concealment, blinding of participants and caregivers, blinding for outcome assessment, incomplete outcome, selective outcome reporting and other potential bias.25 Any disagreements between reviewers in study inclusion, data extraction, and quality assessment that could not be resolved by consensus were resolved by a third reviewer (RB). All analyses were conducted using the statistical software Review Manager (v5.2).
RESULTS
Identification of studies
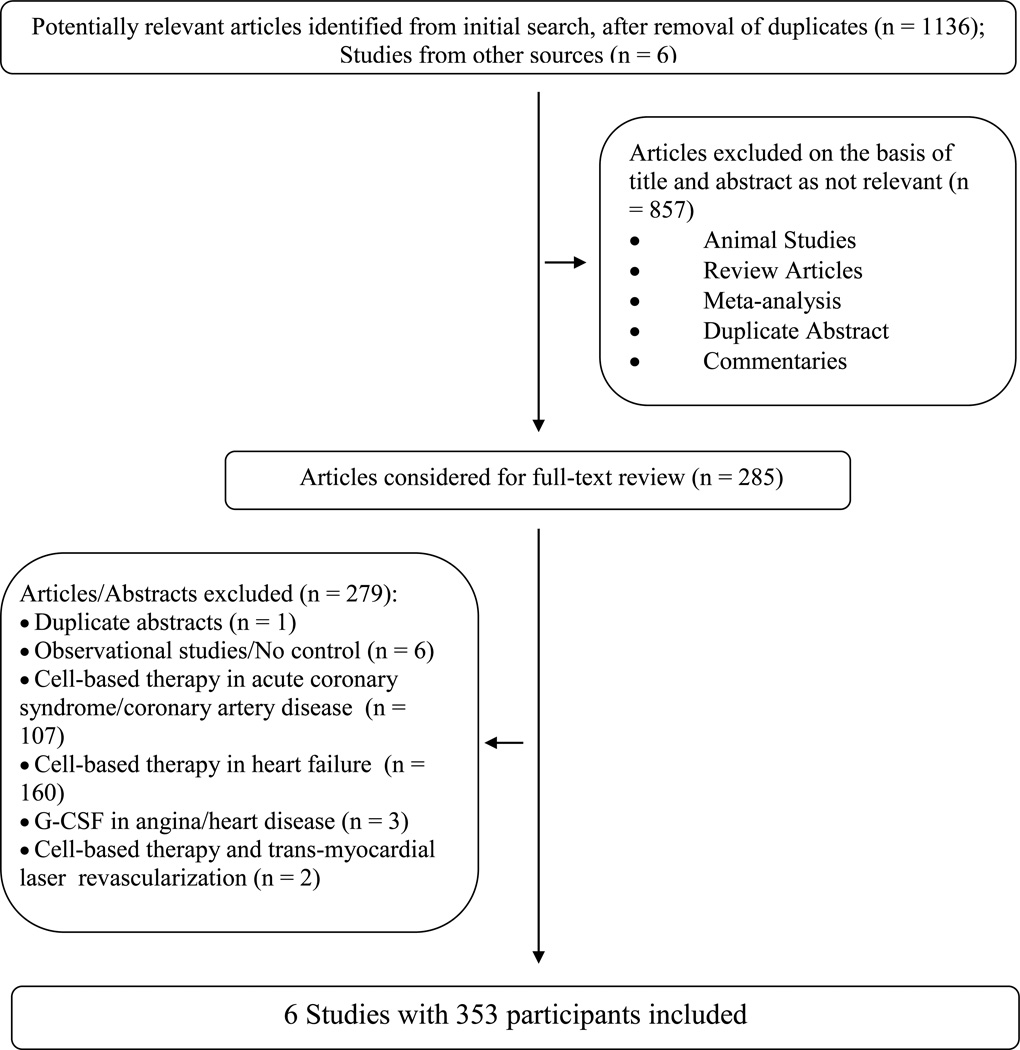
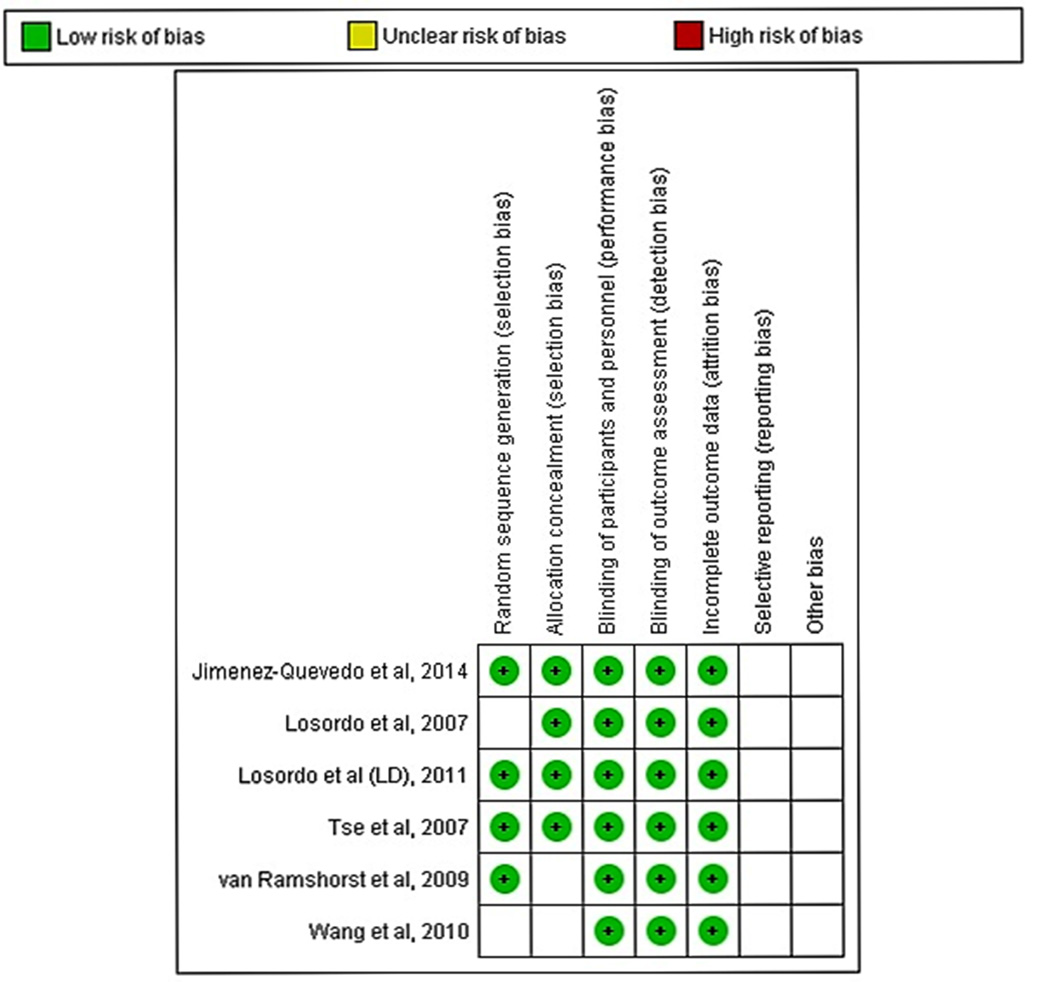
The literature search identified 1,136 publications, out of which six studies were eligible for our analysis (Fig. 1).14–19 To reduce variability in the study population, studies of ischemic cardiomyopathy that did not report angina symptoms refractory to medical therapy were not included.26–29 One study reported outcomes with two different doses of cell-based therapy;18 to avoid duplication, only the dose (low dose group – LD) reported to have better outcome was included in the analysis. The risk of bias in all included studies was determined to be low because the studies were of sound methodological quality (Fig. 2). There was no allocation bias, since adequate randomization was reported in all of the trials except two.14, 17 All assessments of outcomes measured were blinded, with a low risk of documented bias both for selection and for reported outcomes. The RCTs included in the analysis had a low risk of bias due to attrition during follow-up. There was excellent agreement between the reviewers with respect to inclusion of the studies, data abstraction, and quality assessment.
Figure 1.
Flowchart of eligible studies
Figure 2.
Risk of bias of the included studies
Study characteristics
Table 1 summarizes the characteristics of the included studies. Table 2 highlights the characteristics of the patient populations in these studies. A total of six trials comprising 353 participants (ranging from 24-112 in individual trials) were included in the analysis. The studies were conducted in centers located in the United States,14, 18 Europe,16, 19 Asia15, 17 and Australia.15 Four studies were carried out in more than one center.14, 15, 18, 19
Table 1.
Characteristics of Included Studies
| Study; Country |
Study Design |
Center | Inclusion Criteria | Exclusion Criteria | Cell Type; Route of Delivery |
Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| Losordo;14 USA |
RCT: double- blind, placebo- controlled |
3 | Age >21 yr, CCS III/IV, refractory to medical therapy, 2 anti-anginals, ineligible for revascularization, documented ischemia on nuclear perfusion |
MI ≤ 30 days, revascularization ≤ 3 months, joint disease/COPD/PVD which may limit walking on treadmill severe AS/MS, CHF symptoms, life expectancy < 1 year, uncontrolled HTN |
CD34+ cells, Intramyocardial (EMM, NOGA guided) |
Angina frequency, NTG use, exercise tolerance, CCS class, SPECT imaging, QoL; Safety |
1,2,4 weeks; 2,3,6, 9 and 12 months |
| Tse;15 Hong Kong, Australia |
RCT: blind, placebo- controlled |
2 | CCS III/IV, refractory to medical therapy, ineligible for revascularization, documented viable myocardium on SPECT, |
Unprotected L main, decompensated CHF, LVEF ≤ 30%, ACS or stroke within 3 months, significant renal, liver or hematological abnormalities; AF/AS/LV thrombus/PAD prevent electromechanical LV mapping |
BMMNC; Intramyocard ial (EMM, NOGA guided) |
Exercise treadmill, SPECT, cardiac MRI |
3,6 months |
| Ramshorst;16 Netherlands |
RCT: double- blind, placebo- controlled |
1 | CCS III/IV despite medical therapy, documented ischemia in at least 1myocardial segment on SPECT, ineligible for revascularization |
LVEF < 35%, MI within 6 months, GFR < 30 ml/min, unexplained hematological abnormalities, malignancy |
BMMNC; Intramyocard ial (EMM, NOGA guided) |
CCS class, QoL, exercise capacity, myocardial perfusion, LV function and volumes, arrhythmia |
3,6 months |
| Wang;17 China |
RCT; placebo- controlled |
1 | Age >30 yr, CCS III/IV, refractory to medical therapy, ineligible for revascularization, ischemia on nuclear perfusion, angina during baseline exercise |
MI within 30 days, revascularization within 6 months, TIA within 60 days, severe AS/MS, CHF symptoms, life expectancy < 1 year, uncontrolled HTN; joint disease/COPD/PVD which may limit walking on treadmill |
CD34+ cells; Intracoronary |
Angina frequency, NTG use, exercise tolerance, CCS class, SPECT imaging; Safety |
1,2,4 weeks; 3 and 6 months |
| Losordo;18 USA |
RCT: double- blind, placebo- controlled |
26 | Age >21 yr, CCS III/IV, refractory to medical therapy, angina during baseline exercise, ischemia on nuclear perfusion, ineligible for revascularization |
MI within 60 days, revascularization within 3 months, LVEF < 25%, predominant CHF symptoms, severe AS, prosthetic aortic valve, COPD which may limit walking on treadmill, creatinine > 2.5 mg/dl |
CD34+ cells; Intramyocard ial (EMM, NOGA guided) |
Angina frequency, anti-anginal use, exercise tolerance, CCS class, SPECT imaging, MRI; Safety |
28 days; 3,6 and 12 months |
| Quevedo;19 Spain |
RCT; single- blind, placebo- controlled |
3 | CCS II-IV, refractory to medical therapy, coronary anatomy not amenable to revascularization, documented ischemia on SPECT |
MI within 3 months, LV thrombus, aortic valve ds, hemorrhagic disorder, LV wall thickness < 8 mm, history of malignancy ≤5 years, pregnancy |
CD133+ cells; Intramyocard ial (EMM, NOGA guided) |
CCS class, treadmill test, SPECT, anti- anginal medication; QoL, MRI; Safety |
1 week; 1,3 and 6 months; 1 and 2 years |
Abbreviations: USA, United States; RCT, Randomized controlled trial; CCS, Canadian cardiovascular society functional angina class; MI, Myocardial Infarction; AS, Aortic stenosis; MS, Mitral stenosis; CHF, Congestive heart failure; HTN, Hypertension; COPD, Chronic obstructive pulmonary disease; PVD, Peripheral vascular disease; NTG, Nitroglycerine; SPECT, Single photon emission computed tomography; QoL, Quality of life; BMMNC, Bone marrow mononuclear cells, LV, Left ventricular.
Table 2.
Baseline Characteristics of the Included Patients
| Study | Groups | n | Age (yr) |
Male (%) |
DM (%) |
HTN (%) |
HPL (%) |
Smoking (%) |
CHF (%) |
Prior (%) |
Medications (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI | PCI | CABG | BB | ACEi | CCB | Nitrate | Statin | ||||||||||
| Losordo14 | Cell- treated |
18 | 62 | 80 | 39 | 78 | 94 | 72 | 33 | 50 | 83 | 94 | 94 | 67 | NR | NR | 89 |
| Control | 6 | 50 | 50 | 66 | 83 | 33 | 61 | 100 | 66 | 100 | 50 | NR | NR | 83 | |||
| Tse15 | Cell- treated |
19 | 65 | 79 | 42 | 68 | 100 | 42 | NR | NR | 47 | 68 | 95 | 32 | 42 | 42 | 100 |
| Control | 9 | 69 | 88 | 63 | 75 | 100 | 50 | NR | NR | 88 | 63 | 88 | 50 | 50 | 50 | 100 | |
| Ramshorst16 | Cell- treated |
25 | 64 | 92 | 52 | 48 | 48 | 40 | NR | 56 | 64 | 96 | 96 | 76 | 72 | 84 | 100 |
| Control | 25 | 62 | 80 | 32 | 44 | 60 | 48 | NR | 72 | 52 | 76 | 96 | 56 | 72 | 84 | 100 | |
| Wang17 | Cell- treated |
56 | NR | NR | 45 | 39 | 55 | 20 | 05 | NR | 05 | 03 | 64 | 71 | 68 | 70 | 52 |
| Control | 56 | NR | NR | 50 | 43 | 57 | 21 | 07 | NR | 04 | 02 | 64 | 71 | 66 | 71 | 54 | |
| Losordo18 | Cell- treated |
55 | 61 | 84 | 47 | 95 | NR | 75 | 22 | 78 | 87 | 93 | 91 | 76 | 42 | 66 | 76 |
| Control | 56 | 62 | 89 | 55 | 95 | NR | 73 | 41 | 75 | 84 | 96 | 98 | 77* | 52 | 73 | 70 | |
| Quevedo19 | Cell- treated |
19 | 70 | 79 | 52 | 84 | 89 | 15 | NR | 68 | 90 | NR | 95 | 90 | 42 | 84 | 100 |
| Control | 9 | 58 | 100 | 55 | 100 | 66 | 11 | NR | 67 | 78 | NR | 78 | 78 | 67 | 89 | 100 | |
Abbreviations: DM, Diabetes mellitus; HTN, Hypertension; HPL, Hyperlipidemia; CHF, Congestive heart failure; MI, Myocardial Infarction; PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass grafting; BB, Beta blocker; ACEi, Angiotensin converting inhibitor enzyme inhibitor; CCB, Calcium channel blocker
Use of both ACEi and angiotensin receptor blocker
The patients included in the individual studies had symptoms of angina, CCS III-IV, refractory to medical therapy and were not candidates for coronary revascularization. The patients were predominantly male with an average age >60 years in almost all studies. In the study by Wang and colleagues,17 the patient population in the cell-based therapy group was relatively older as compared with the control group. In all studies except that by Wang et al,17 the patient population had a high incidence of prior PCI and CABG (Tables 1 and 2).
There were 192 patients who received cell therapy along with the current standard of care and 161 patients who were on maximal medical therapy. Three studies used CD34+ cells,14, 17, 18 two unfractionated bone marrow mononuclear cells (BMMNC)15, 16, and one CD133+ cells.19 The techniques to enrich and harvest the cells differed. Some studies used granulocyte colony stimulating factor (G-CSF) to treat patients prior to harvest.14, 17–19 Three studies harvested the cells directly from the bone marrow15–17 while three studies harvested the cells from peripheral blood.14, 18, 19 Three studies used magnetic sorting of cells14, 18, 19 while two studies used density gradient centrifugation15, 16 to enrich the cells after harvesting them. All14–16, 18, 19 but one study17 used electro-mechanical mapping with the NOGA navigation system to deliver cells to the myocardium. In one study the cells were infused into the left main and right coronary arteries during cardiac catheterization.17 (Tables 1 and 2).
Efficacy of cell-based therapy
The efficacy of cell-based therapy was assessed by measuring changes in perfusion of the ischemic myocardium, changes in the indices of angina, and the composite cardiovascular end-point. The indices used for the assessment of angina were frequency of angina episodes, CCS class, use of anti-anginal medications, exercise tolerance, and quality of life.
Myocardial perfusion
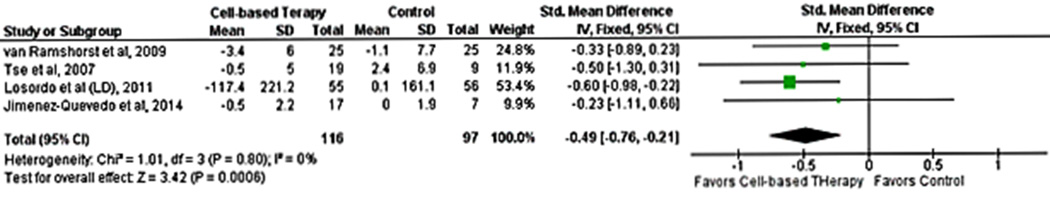
SPECT was the imaging modality used to assess changes in myocardial perfusion, which were measured as the difference in SPECT scores between end of follow-up and baseline. Studies reported (i) both summed stress and summed rest scores,16, 19 (ii) both summed stress and difference scores,15 (iii) summed stress18 only, or (iv) summed difference score14 only. Overall, four studies reported the change in summed stress scores,15, 16, 18, 19 two reported the change in summed rest scores,16, 19 and two reported the change in summed difference scores14, 15. All studies evaluated perfusion at 6 months except two studies, one of which reported SPECT scores both at 3 and 6 months14 while the other reported SPECT scores at 3 months16 (Supplementary Table I). One study reported both automated and visually estimated scores14; the scores with the lower reported improvement were included in the analysis. Given the limited number of studies that reported summed rest or difference scores, we pooled only summed stress scores to assess perfusion.
Cell-based therapy was associated with an improvement in myocardial perfusion as measured by summed stress score (SMD -0.49;95% CI, -0.76−0.-21, P=0.0006; I2=0%). There was no between-study heterogeneity (I2=0%) (Fig. 3). Publication bias was not assessed because of the limited number of studies in the analysis.
Figure 3.
Forest plot showing the weighted differences between the mean changes from baseline in myocardial perfusion (measured by SPECT) in patients with refractory angina who received cell-based therapy compared with maximal medical therapy.
Anginal episodes
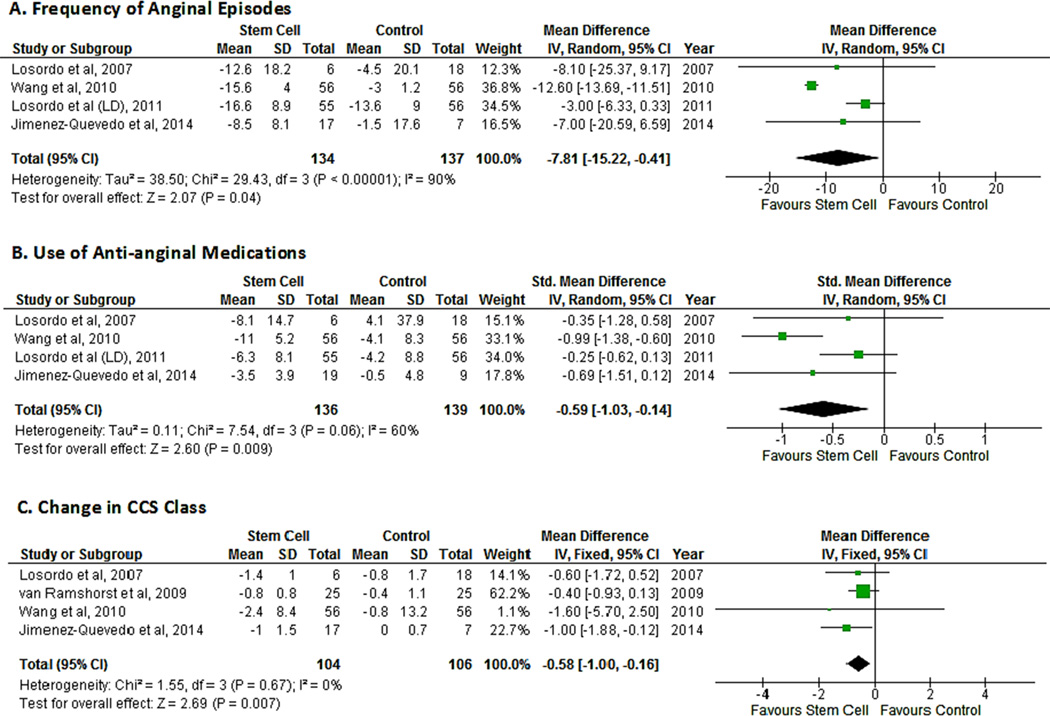
The mean change in frequency of anginal episodes vs. baseline was reported in four studies.14, 17–19 The pooled analysis of the studies suggested an improvement in the number of angina episodes in patients treated with cell therapy (MD -7.81; 95% CI, -15.25−0.-37, P=0.04). There was substantial between-study heterogeneity (I2=90%) and sensitivity analysis revealed that all the heterogeneity was secondary to one study.17 After removal of that study, heterogeneity dropped to 0% and the MD decreased as well (MD -3.38;95% CI, -6.56−0.-19, P=0.04) (Fig. 4A).
Figure 4.
Forest plot showing the weighted differences (MD or SMD) between the mean changes from baseline in indices of angina in patients with refractory angina who received cell-based therapy compared with maximal medical therapy. A. Mean difference in the frequency of angina episodes. B. Standardized mean difference in use of anti-anginal medications. C.Mean difference in CCS class.
Changes in anti-anginal medications
The mean change in the number of medications vs. baseline was reported in four studies.14, 17–19 Two studies reported mean changes in medications per week14, 17 while one study each reported mean changes per day18 or per month.19 A meta-analysis of these four studies suggested a decrease in the use of anti-anginal medications in the cell therapy group (SMD -0.62; 95% CI, -1.05−0.-18, P=0.006; I2=59%). Sensitivity analysis revealed that all of the heterogeneity was secondary to one study.17 After removal of that study, heterogeneity dropped to 0% and the SMD decreased as well (SMD -0.35, 95% CI, -0.67−0.-03, P=0.03) (Fig. 4B).
Change in CCS class
The mean change in functional class vs. baseline was reported in all included studies except one.15 However, one study reported improvement in number of patients rather than a change in class.18 A pooled analysis of four studies suggested an improvement in CCS class in patients who received cell therapy (MD -0.58; 95% CI, -1.00−0.-16, P=0.007; I2=0%) (Fig. 4C).
Change in exercise tolerance
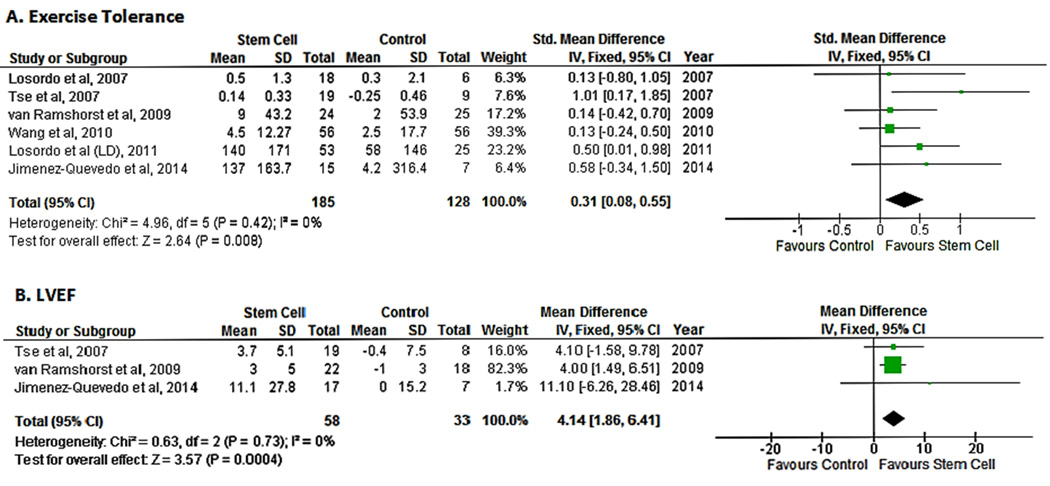
The mean change in exercise tolerance vs. baseline was reported in all studies. Exercise capacity was measured by a treadmill test with the standard Bruce protocol in two studies,14, 17 a treadmill test with a modified Bruce protocol in two studies,15, 18 and a symptom-limited bicycle exercise test in one study.16 One study used a treadmill test to ascertain tolerance but did not report the protocol used.19 Pooled analysis of the included studies demonstrated an improvement in exercise tolerance in the cell therapy group as compared with the control population (SMD 0.31;95% CI, 0.08−0.55, P=0.008; I2=0%)(Fig. 5A).
Figure 5.
Forest plot showing the weighted difference (MD or SMD) between the mean changes from baseline in functional and cardiovascular outcomes in patients with refractory angina who received cell-based therapy compared with maximal medical therapy. A. Standardized mean difference in exercise tolerance. B. Mean difference in left ventricular ejection fraction (LVEF).
Left ventricular function
Three studies reported measurements of LVEF.15, 16, 19 LVEF was measured by cardiac MRI in two studies15, 16 and by three modalities, SPECT, echocardiography and ventriculography, in one study.19 Pooled data from the three studies suggested an improvement in LV function in the cell therapy group as compared with controls (SMD 4.14; 95% CI, 1.86−6.41, P=0.004; I2=0%)(Fig. 5B).
Quality of life
Quality of life was assessed with the Seattle Angina Questionnaire in four studies, all of which reported an improvement in the cell therapy group.14, 16, 18, 19 One study reported improvement in four of the five parameters measured but did not report the mean changes in scores14. One study reported the angina stability scale from the questionnaire but did not report any other parameter.18 We did not conduct a pooled analysis of the quality of life measures because different scales were used and incomplete data were reported. However, all studies reported an improvement in quality of life measures.
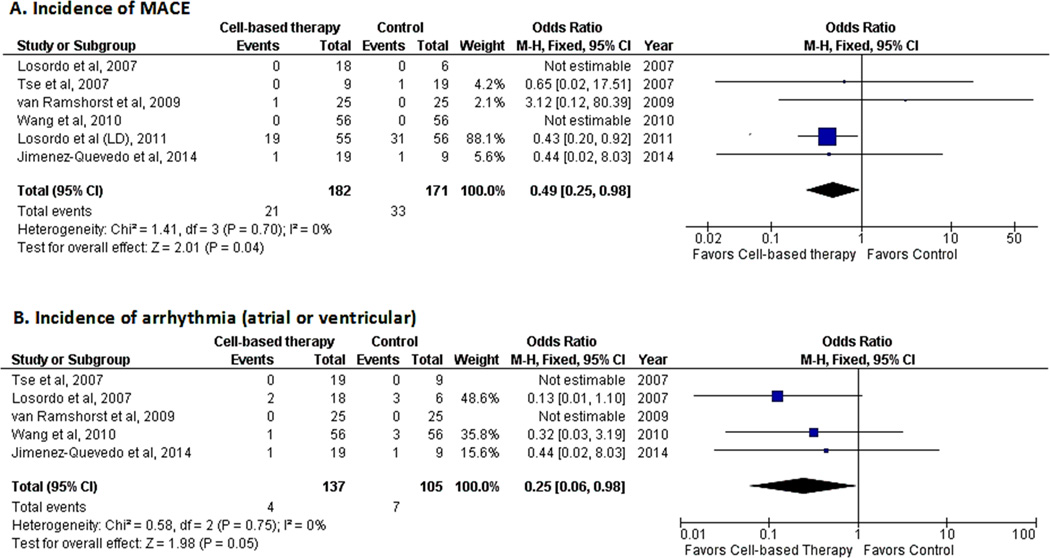
Composite cardiac end-point
All included studies analyzed myocardial infarction or mortality either as a clinical outcome or as a safety measure. Cardiac-related hospitalization was reported in two studies.18, 19 The occurrence of myocardial infarction was reported in two studies,15, 18 cardiac-related hospitalization in two studies,18, 19 and mortality in three studies.16, 18, 19 The composite cardiac end-point of all of these outcomes suggested a decreased risk of occurrence of MACE (OR 0.49; 95% CI, 0.25−0.98, P=0.04; I2=0%)(Fig. 6A) in patients who received cell-based therapy.
Figure 6.
Forest plot showing clinical outcomes in patients who received cell-based therapy compared with maximal medical therapy. A. Incidence of major adverse cardiovascular events (MACE). B. Incidence of arrhythmias (atrial or ventricular).
Safety
All included studies evaluated the safety of cell-based therapy. Adverse events reported during follow-up were mortality, congestive heart failure, angina exacerbation, respiratory arrest, tumor occurrence, bleeding, renal insufficiency, and arrhythmias. With the exception of MACE (vide supra) and arrhythmias (atrial and ventricular) (vide infra), the other adverse events were either not consistently evaluated in all studies or not found to differ between the two groups; therefore, no meta-analyses were performed for these safety parameters.
Five studies evaluated the occurrence of arrhythmias (both atrial and ventricular).14–17, 19 A pooled analysis revealed a decreased risk of arrhythmias in the cell-based therapy group (OR 0.25; 95% CI, 0.06−0.98, P=0.05; I2=0%)(Fig. 6B) as compared with maximal medical therapy. Analysis of the composite cardiac end-point was also a measure of safety and, as indicated above, suggested a decreased risk of MACE in the cell-based therapy group (Fig. 6A).
DISCUSSION
Administration of stem/progenitor cells is a new therapeutic approach with immense potential. In this meta-analysis of patients with angina refractory to medical therapy and ineligible for revascularization, we found a significant improvement in several indices of angina, namely, decreased frequency of angina episodes, improvement in CCS class, and decreased use of anti-anginal medications in cell-treated patients. These clinical changes were associated with an improvement in myocardial perfusion (as demonstrated by SPECT imaging), exercise tolerance, and left ventricular EF and a marked decrease in MACE. Moreover, cell-based therapy was found to be safe. The present work is the largest and most current meta-analysis of cell therapy trials in RFA to date; the results provide strong evidence supporting a beneficial effect of cell-based treatments and a robust rationale for larger, definitive phase III trials.
The importance of this analysis stems from the fact that, although the individual studies of RFA conducted heretofore reported a favorable trend, they were limited by their small sample size and by the use of different primary end-points. As a result, the effect of stem/progenitor cells in this patient population is unclear. The present analysis advances the field because it indicates that when all available RCTs are pooled and the same end-points are evaluated across studies, cell therapy has a statistically significant, beneficial effect not only on indices of angina and myocardial perfusion, but also on clinical outcomes.
Although our analysis revealed an improvement in indices of angina, exercise tolerance, left ventricular function, and myocardial perfusion in cell-treated patients, it did not demonstrate a reduction in mortality. An effect of cell therapy on mortality, however, would be difficult to detect because the studies included in this analysis were not large enough to assess this end-point; they were designed to establish safety and provide initial evidence of symptomatic efficacy, not to show a decrease in mortality. Moreover, mortality in patients with RFA is low5, 30, 31 making it difficult to demonstrate a significant change even when the six studies were pooled (total of 353 patients). Nevertheless, our analysis does suggest a decreased risk of MACE in patients who received cell therapy (Figure 6A). The reduction in MACE in the absence of significant changes in mortality in this study is consistent with a recent analysis of a database of patients with RFA, which has shown a low mortality but a relatively high incidence of a composite of death, MI, and cardiac-related hospitalizations in this population.5 Together with the results of that study,5 the present investigation suggests that measures of morbidity and MACE are more appropriate end-points than mortality when designing trials in this patient population.
The mechanism whereby cell therapy produces clinical improvement in patients with refractory angina is unclear. Several lines of evidence suggest that the salubrious effects of cell therapy are secondary to paracrine actions of transplanted cells that promote neovascularization and collateral perfusion.8, 12 Even in the setting of a compromised macrovascular supply, improvement in microvascular and collateral perfusion can augment contractile function.32, 33 The concept that cell therapy promotes neovascularization is supported by our finding that, in treated patients, there was an increase in myocardial perfusion, as assessed by SPECT (Fig. 3). Although SPECT is a good tool to detect changes in myocardial perfusion, its ability to do so may be limited in this patient population because the frequent presence of multivessel disease reduces the relative magnitude of changes in perfusion. Despite these limitations, however, our analysis was able to show a beneficial effect of cell therapy on myocardial perfusion, as evidenced by the changes in summed stress scores. An abnormal perfusion scan has been reported to be a surrogate measure for adverse clinical outcomes.34
Compared with a previous meta-analysis of cell therapy in RFA,21 our study is based on a comprehensive literature search that includes the largest number of relevant studies to date. Our study expands on that previous study21 by including more functional and clinical end-points, by demonstrating improved myocardial perfusion, and by assessing the effect of cell-based therapy on a composite clinical cardiovascular endpoint. Thus, the present work adds substantially to the existing evidence in support of cell therapy in RFA.
Some limitations of the present analysis need to be discussed. The robustness of the evidence suggesting a beneficial effect of cell therapy was negatively affected by the small sample sizes and, in some cases, the short (<1 year) follow-up periods of the studies included, as well as by the variance in the results of individual studies. This variation may be partly explained by differences in cell type, cell dosage, cell isolation protocols, cell delivery methods, perfusion scores measured, and definition of successful outcome, as well as by the heterogeneity encountered in some of the analyses. All of the heterogeneity was accounted for by one study,17 which differed from the other studies with respect to patients’ baseline characteristics, co-existing diseases, need for prior coronary revascularization, and use of concomitant medications. Removal of that study affected the magnitude of the beneficial effect of cell-based therapy but did not change the direction of the results, which remained in favor of the treated group.
It should be pointed out that although the present study supports the utility of stem/progenitor cell administration in RFA, meta-analyses cannot demonstrate therapeutic efficacy. Larger phase III trials are needed to provide definitive evidence, evidence sufficient to lead to FDA approval and routine use of cell-based therapy in this clinical setting. Nonetheless, when evaluating the role of cell-based therapy in RFA, the lessons learned from the early-phase clinical trials reviewed herein will be important for designing future studies. These phase I/II studies used either cells that expressed markers of EPCs (CD34+ and CD133+) or unfractionated BMMNCs. Based on current evidence, both of these cell types show promise, and it would be difficult to formulate a recommendation as to which type should be tested further. MSCs could be another option, as they have been reported to have a beneficial effect in RFA even when used alone35 and can be obtained from healthy donors and used in an allogeneic manner because of their immunoprivileged status.36
Furthermore, future studies should be designed to include standardized quantitative assessment of myocardial perfusion, quality of life measures, and MACE as measures of efficacy. Although most of the included studies used SPECT imaging to assess changes in myocardial perfusion, this method is limited by poor spatial resolution, long acquisition protocols and, most importantly, the occurrence of balanced flow reduction (e.g., in multivessel disease). Other available modalities, such as positron emission tomography (PET) and cardiac magnetic resonance imaging (CMR), can be used with better capabilities to detect regional and global myocardial perfusion. It would seem more appropriate to use multimodality imaging with a combination of PET and CMR to assess both anatomical and functional changes after cell-based therapy. In addition to objective evidence of changes in myocardial perfusion, it is important to demonstrate improvement in clinical outcomes. Relevant measures of efficacy should be not only indices of angina but also quality of life measures. Moreover, as mentioned above, a composite cardiac end-point that includes cardiac mortality, myocardial infarction, and cardiac-related hospitalization would be most useful to assess efficacy.
In conclusion, the clinical trials of cell therapy conducted heretofore in patients with angina refractory to medical treatment and not amenable to revascularization (no-option angina) are limited by the small sample size and, in some cases, the short-follow-up period, making it difficult to discern an efficacy signal. Individually, these studies have been mostly inconclusive. These limitations can be overcome, in part, by a meta-analysis. The present analysis, based on a total of 353 patients from six RCTs, suggests that cell-based therapies are not only safe, but also lead to an improvement in indices of angina, exercise tolerance, left ventricular function, and myocardial perfusion and a decrease in MACE. These encouraging results provide a strong rationale for conducting larger, rigorous RCTs to conclusively determine the efficacy of cell-based therapy in this problematic patient population in which few, if any, options are currently available.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Cell-based therapies have the potential to promote neovascularization and endothelial repair and consequently myocardial perfusion.
Several phase I-II studies have been conducted to assess the effect of cell therapy in patients with angina refractory to conventional medical therapy and ineligible for coronary revascularization (refractory or no-option angina).
These early phase I-II studies are limited by their small size and lack of uniform primary end-points and clinical outcomes, making it difficult to discern an efficacy signal.
There is no phase III trial currently underway; thus, the effect of cell-based therapies on myocardial perfusion and clinical outcomes in Refractory Angina remains unclear.
What New Information Does This Study Contribute?
When the same end-points are evaluated across studies, cell therapy has a significant beneficial effect not only on indices of angina and myocardial perfusion, but also on clinical outcomes.
As none of the studies performed thus far was powered to assess clinical end-points, we combined the cardiovascular outcomes into a composite cardiac end-point (MACE); our analysis shows that cell-based therapies lead to an improvement in clinical outcomes as demonstrated by a reduction in MACE.
Our results provide strong evidence supporting a beneficial effect of cell-based treatments and a robust rationale for larger, definitive phase III trials.
Cell-based therapies have shown safety and efficacy in several early proof-of-concept studies. However, these studies, while showing safety and efficacy, are limited because they evaluated different end-points and also were not powered enough to assess clinical outcomes. Results of our meta-analysis suggest that cell-based therapies lead to improvement not only in myocardial perfusion but also in functional indices of angina and to reduction in a composite cardiac endpoint that includes myocardial infarction, cardiac-related hospitalization, and mortality. Although the present analysis supports the utility of cell-based therapies in ischemic heart disease and refractory angina, it cannot demonstrate therapeutic efficacy. Larger phase III studies are needed to provide definitive evidence of cell therapy in patients with Refractory Angina.
Acknowledgments
We acknowledge the help of Michel Atlas in the development of the search strategy and the literature search.
SOURCES OF FUNDING
This work was supported in part by NIH grants P01 HL078825, P20 GM103492, and UM1 HL113530.
Nonstandard Abbreviations and Acronyms
- RFA
refractory angina
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- EPC
endothelial progenitor cells
- SPECT
Single photon emission computed tomography
- MD
Mean difference
- SMD
Standardized mean difference
- CCS
Canadian Cardiovascular Society
- MACE
Major adverse cardiac events
- BMMNC
Bone marrow mononuclear cells
- G-CSF
granulocyte colony stimulating factor
- LVEF
Left Ventricular Ejection Fraction
- PET
positron emission tomography
- CMR
cardiac magnetic resonance imaging
Footnotes
DISCLOSURES.
The authors have declared that no competing interests exist.
REFERENCES
- 1.McGillion M, Arthur HM, Cook A, Carroll SL, Victor JC, L'Allier PL, Jolicoeur EM, Svorkdal N, Niznick J, Teoh K, Cosman T, Sessle B, Watt-Watson J, Clark A, Taenzer P, Coyte P, Malysh L, Galte C, Stone J Canadian Cardiovascular S, Canadian Pain S. Management of patients with refractory angina: Canadian cardiovascular society/canadian pain society joint guidelines. The Canadian journal of cardiology. 2012;28:S20–S41. doi: 10.1016/j.cjca.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis--how many patients might be eligible? The American journal of cardiology. 1999;84:598–600. A598. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 3.Henry TD, Satran D, Jolicoeur EM. Treatment of refractory angina in patients not suitable for revascularization. Nature reviews. Cardiology. 2014;11:78–95. doi: 10.1038/nrcardio.2013.200. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circulation research. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Povsic TJ, Broderick S, Anstrom KJ, Shaw LK, Ohman EM, Eisenstein EL, Smith PK, Alexander JH. Predictors of long-term clinical endpoints in patients with refractory angina. Journal of the American Heart Association. 2015;4 doi: 10.1161/JAHA.114.001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin F, Al Hajeri A, Civelek B, Fedorowicz Z, Manzer BM. Enhanced external counterpulsation for chronic angina pectoris. The Cochrane database of systematic reviews. 2010:CD007219. doi: 10.1002/14651858.CD007219.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briones E, Lacalle JR, Marin-Leon I, Rueda JR. Transmyocardial laser revascularization versus medical therapy for refractory angina. The Cochrane database of systematic reviews. 2015;2:CD003712. doi: 10.1002/14651858.CD003712.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation research. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 9.Jolicoeur EM, Granger CB, Henry TD, Holmes DJ, Pepine CJ, Mark D, Chaitman BR, Gersh BJ, Ohman EM, Working Group M. Clinical and research issues regarding chronic advanced coronary artery disease: Part i: Contemporary and emerging therapies. American heart journal. 2008;155:418–434. doi: 10.1016/j.ahj.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Jolicoeur EM, Ohman EM, Temple R, Stockbridge N, Smith S, Mark D, Califf RM, Henry TD, Chaitman BR, Granger CB, Working Group M. Clinical and research issues regarding chronic advanced coronary artery disease part ii: Trial design, outcomes, and regulatory issues. American heart journal. 2008;155:435–444. doi: 10.1016/j.ahj.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 12.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 13.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circulation research. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous cd34+ stem cells for intractable angina: A phase i/iia double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 15.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (protect-cad trial) European heart journal. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 16.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Cui J, Peng W, Lu M. Intracoronary autologous cd34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217. [DOI] [PubMed] [Google Scholar]
- 18.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA, Investigators AC. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, Garcia-Moll X, Delgado-Bolton R, Llorente L, Bernardo E, Ortega-Pozzi A, Hernandez-Antolin R, Alfonso F, Gonzalo N, Escaned J, Banuelos C, Regueiro A, Marin P, Fernandez-Ortiz A, Neves BD, Del Trigo M, Fernandez C, Tejerina T, Redondo S, Garcia E, Macaya C. Selected cd133(+) progenitor cells to promote angiogenesis in patients with refractory angina: Final results of the progenitor randomized trial. Circulation research. 2014;115:950–960. doi: 10.1161/CIRCRESAHA.115.303463. [DOI] [PubMed] [Google Scholar]
- 20.Povsic TJJC, Nada A, Schatz RA, Harrington RA, Davidson CJ, Fortuin FD, Kereiakes DJ, Mendelsohn FO, Sherman W, Schaer GL, White CJ, Stewart D, Story K, Losordo DW, Henry TD. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Efficacy and safety of targeted intramyocardial delivery of auto cd34+ stem cells for improving exercise capacity in subjects with refractory angina (renew) [Last updated 2015/05/21; Last accessed 2015/10/26]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01508910?term=refractory+angina+OR+no-option+angina+AND+stem+cell+therapy+OR+cell-based+therapy&rank=5. [Google Scholar]
- 21.Li N, Yang YJ, Zhang Q, Jin C, Wang H, Qian HY. Stem cell therapy is a promising tool for refractory angina: A meta-analysis of randomized controlled trials. The Canadian journal of cardiology. 2013;29:908–914. doi: 10.1016/j.cjca.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPTGS. The cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated march 2011) The Cochrane Collaboration. 2011 [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G Cochrane Statistical Methods G. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD, Traverse JH, Zheng Y, Smith D, Shaw S, Westbrook L, Olson R, Patel D, Gahremanpour A, Canales J, Vaughn WK, Willerson JT. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (focus-hf) American heart journal. 2011;161:1078–1087. e1073. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Perin EC, Silva GV, Zheng Y, Gahremanpour A, Canales J, Patel D, Fernandes MR, Keller LH, Quan X, Coulter SA, Moore WH, Herlihy JP, Willerson JT. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. American heart journal. 2012;163:415–421. 421–e411. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The focus-cctrn trial. JAMA : the journal of the American Medical Association. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pokushalov E, Romanov A, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, Poveshenko O, Kliver E, Shirokova N, Karaskov A, Dib N. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: A randomized study. Journal of cardiovascular translational research. 2010;3:160–168. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 30.Henry TD, Satran D, Hodges JS, Johnson RK, Poulose AK, Campbell AR, Garberich RF, Bart BA, Olson RE, Boisjolie CR, Harvey KL, Arndt TL, Traverse JH. Long-term survival in patients with refractory angina. European heart journal. 2013;34:2683–2688. doi: 10.1093/eurheartj/eht165. [DOI] [PubMed] [Google Scholar]
- 31.Williams B, Menon M, Satran D, Hayward D, Hodges JS, Burke MN, Johnson RK, Poulose AK, Traverse JH, Henry TD. Patients with coronary artery disease not amenable to traditional revascularization: Prevalence and 3-year mortality. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2010;75:886–891. doi: 10.1002/ccd.22431. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of cd34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 33.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 34.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 35.Haack-Sorensen M, Friis T, Mathiasen AB, Jorgensen E, Hansen L, Dickmeiss E, Ekblond A, Kastrup J. Direct intramyocardial mesenchymal stromal cell injections in patients with severe refractory angina: One-year follow-up. Cell transplantation. 2013;22:521–528. doi: 10.3727/096368912X636830. [DOI] [PubMed] [Google Scholar]
- 36.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell death and differentiation. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.