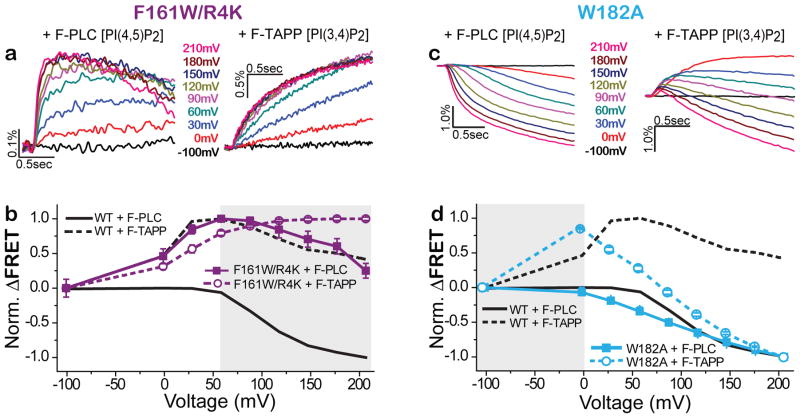
Figure 5. VSD mutants stabilize discrete enzyme activity states.
a,b) F161W/R4K mutant favors the A1 (PIP3→PIP2) enzyme activity state. F-PLC detects that F161W/R4K (a, traces; b, solid squares, n=14) augments the accumulation of PI(4,5)P2 (increased FRET due to dephosphorylation of the 3-position phosphate of PI(3,4,5)P3) and suppresses the depletion of PI(4,5)P2 (decreased FRET due to 5-position dephosphorylation of PI(4,5)P2) that are characteristic of WT Ci-VSP (solid grey line). F-TAPP detects that F161W/R4K (a, traces; b, open circles, n=12) augments the accumulation of PI(3,4)P2 (increased FRET due to dephosphorylation of the 5-position phosphate of PI(3,4,5)P3) and suppresses the depletion of PI(3,4)P2 (decreased FRET due to 5-position dephosphorylation of PI(3,4,5)P3) that are characteristic of WT Ci-VSP (dashed grey line). c,d) W182A shifts to more negative voltage the transition from the inactive state to the A1 active state that produces accumulation of PIP2 as well as between A1 and the A2 state that depletes of PIP2, as seen in both F-PLC (c, traces; d, solid squares, n=12) and F-TAPP (c, traces; d, open circles, n=14).