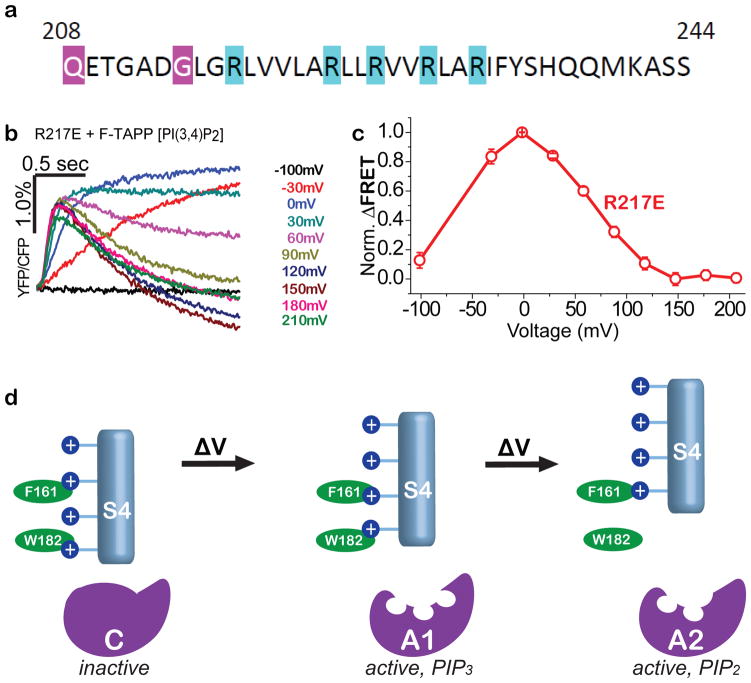
Figure 6. Two-step VSD conformational control over VSP phosphatase with two active states.
a) S4 sequence of with arginines (blue), including R217, whose mutation to glutamate stabilizes an activated conformation of the VSD for crystallography43,67. b,c) F-TAPP traces (b) and F-V (c) shows that the R217E mutant is at peak A1 PI(3,4,5)P3→PI(3,4)P2 activity at zero voltage, suggesting that “up” VSD structure in Fig. 4d corresponds to A1 enzyme state. d) Model of sequential depolarization-driven transitions in VSD that sequentially transition the phosphatase domain from inactive to the PIP3-preferring A1 active state and then to the PIP2-prefering A2 active state.